Abstract
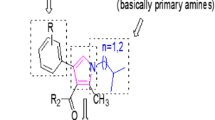
In a search for α-amylase and α-glucosidase inhibitors to treat type II diabetes, a new series of N-(3-acetyl-2-methyl-4-phenylquinolin-6-yl)arylamides were synthesized from 3-acetyl-2-methyl-4-phenylquinolines. Initially, nitro function of 1-(2-methyl-6-nitro-4-phenylquinolin-3-yl) ethanone was converted into the corresponding amine by grinding it with zinc dust and ammonium chloride (reducing agent) which in turn successfully converted into the N-(3-acetyl-2-methyl-4-phenylquinolin-6-yl) arylamides by treating it with coupling reagents such as EDC, HATU, and DCC. All the synthesized compounds were found to afford excellent yields with HATU, moderate in EDC, and very less in DCC and hence, HATU was considered as a suitable coupling reagent. These analogs are structurally characterized by NMR, NMR-DEPT, and HRMS. All the synthesized compounds were evaluated for in-silico and in-vitro α-glucosidase and α-amylase inhibitory activity using acarbose as standard and all the compounds showed positive results by in-silico and in-vitro α-amylase inhibition assay. Among the tested compounds, compound 5c and 5d in α-glucosidase as well as in α-amylase are found to have least binding energy value. These compounds found to form more stable ligand–receptor complex amongst other compounds. In addition, in experimental part, also the compounds 5c and 5d exhibited 56.90 ± 0.77% and 59.46 ± 0.61% of the higher potent α-glucosidase inhibitory activity with IC50 values 171.75 ± 3.95 µmol/mL and 171.67 ± 1.57 µmol/mL significantly (p < 0.05) compared to the remaining seven test samples. And similarly, the compound 5c and 5d possessed α-amylase inhibitory activity at a concentration of 100 µg/mL (55.42 ± 0.42% and 55.42 ± 1.14%) with IC50 values 138.92 ± 4.44 µmol/mL and 158.78 ± 2.34 µmol/mL.







Similar content being viewed by others
References
B. Shori, J. Food. Drug. Anal. 23, 609–618 (2014)
S.A. Adefegha, G. Oboh, O.M. Adefegha, A.A. Boligon, M.L. Athayde, J. Sci. Food Agric. 94, 2726–2737 (2014)
Z. Gong, Y. Peng, J. Qiu, A. Cao, G. Wang, Z. Peng, Molecules 22, 1555–1566 (2017)
S.R. Joshi, E. Standl, N. Tong, P. Shah, S. Kalra, R. Rathod, Expert Opin. Pharmacother. 16, 1959–1981 (2015)
R. Pili. J. Chang, R.A. Partis, R.A. Mueller, F.J. Chrest, A. Passaniti, Cancer Res. 55, 2920–2926 (1995)
J. Rawlings, H. Lomas, A.W. Pilling, M.J.R. Lee, D.S. Alonzi, J.S.S. Rountree, S.F. Jenkinson, G.W.J. Fleet, R.A. Dwek, J.H. Jones, Chem. Bio. Chem. 10, 1101–1105 (2009)
N. Zitzmann, A.S. Mehta, S. Carrouée, T.D. Butters, F.M. Platt, J. McCauley, B.S. Blumberg, R.A. Dwek, T.M. Block, Proc. Natl. Acad. Sci. USA 96, 11878–11882 (1999)
A.K. Ghose, V.N. Viswanadhan, J.J. Wendoloski, J. Combinatorial Chem. 1, 55–68 (1999)
A.A. Bekhit, O.A. El-Sayed, E. Aboulmagd, J.Y. Park, Eur. J. Med. Chem. 39, 249–255 (2004)
L.M. Beal, B. Liu, W. Chu, K.D. Moeller, Tetrahedron 56, 10113–10125 (2000)
C.A. Montalbetti, V. Falque, Tetrahedron 61, 10827–10852 (2005)
L.C. Chan, B.G. Cox, J. Org. Chem. 72, 8863–8869 (2007)
N. Kushwaha, R.M. Salini, K.S. Kushwaha, Int. J. Chem. Tech 3, 204–209 (2011)
V. Duraipandiyan, S. Ignacimuthu, J. Ethnopharmacol. 123, 494–498 (2009)
S.R. Dorn, H. Vippagunta, C. Matile, J.L. Jaquet, R.G. Vennerstrom, Ridley, Biochem. Pharmacol. 55, 727–736 (1998)
V.R. Shanbhag, A.M. Crider, R. Gokhale, A. Harpalani, R.M. Dick, J. Pharm. Sci. 81, 149–154 (1992)
M. Andreani, A. Granaiola, A. Leoni, R. Locatelli, M. Morigi, Rambaldi, J. Med. Chem. 48, 3085–3089 (2005)
W. Gao, J.Y. Kim, S.N. Chen, S.H. Cho, J. Choi, B.U. Jaki, Y. Jin, D.C. Lakin, E. Lee, S.Y. Lee, J.B. McAipline, J.G. Napolitano, S.G. Franzblau, J.W. Suh, G.F. Fauli, Org. Lett. 16, 6044–6047 (2014)
D. Edmont, R. Rocher, C. Plisson, J. Chenault, Bioorg. Med. Chem. Lett. 10, 1831–1834 (2000)
L. Jyothish Kumar, V. Vijayakumar, Res. Chem. Intermed. 21, 5691–5705 (2017)
S. Bienert, A. Waterhouse, T.A. de Beer, G. Tauriello, G. Studer, L. Bordoli, T. Schwede, Nucleic Acids Res. 45, 313–319 (2017)
G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanne, R.K. Belew, D.S. Goodsell, A.J. Olson, J. Comput. Chem. 30, 2785–2791 (2009)
M.J. Abraham, T. Murtola, R. Schulz, S. Páll, J.C. Smith, B. Hess, E. Lindahl, SoftwareX 1–2, 19–25 (2015)
H.M. Berman, J. Westbrook, Z. Feng, G. Gilliand, T.N. Bhat, H. Weissig, I.N. Shindyaloy, P.E. Bourne, The protein data bank. Nucleic Acids Res. 28, 235–242 (2000)
M. Kontoyianni, L.M. Mantzanidou, D.L. Hadjipavlou, J. Med. Chem. 47, 558–565 (2004)
Y. Kim, Y.K. Jeong, M.H. Wang, W.Y. Lee, H.I. Rhee, Nutrition 21, 756–761 (2005)
C. Hansawasdi, J. Kawabata, T. Kasai, Biosci. Biotechnol. Biochem. 64, 1041–1043 (2000)
Acknowledgements
Authors are thankful to the administration, VIT University, Vellore, India, for providing facilities to carry research work and also thankful to SIF-Chemistry for providing NMR facility. Authors are thankful to University of Hyderabad [Network resource centre (UGC-NRC)] for HRMS facility and also NMR facilities. The authors also thankful to the Division of Animal Biotechnology, Department of Biotechnology, School of Herbal Studies and Nature Sciences, Dravidian University. Author L. Jyothish Kumar is thankful to the VIT University for providing research associate ship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, L.J., Suresh, Y., Rajasekaran, R. et al. Synthesis and exploration of in-silico and in-vitro α-glucosidase and α-amylase inhibitory activities of N-(3-acetyl-2-methyl-4-phenylquinolin-6-yl)arylamides. J IRAN CHEM SOC 16, 1071–1080 (2019). https://doi.org/10.1007/s13738-018-01580-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-01580-4