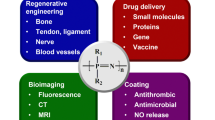
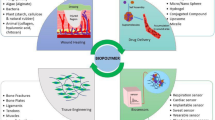
Abstract
Recent advances in biomedicine necessitate the critical and systematic review of current trends in the development and modification of biomaterials for biomedical applications. Synthetic polymers continue to play a leading role as biomedical substrates, and polyphosphazenes, as a promising class of biomaterials, have become a research hotspot in the medical and pharmaceutical fields due to their unique properties, such as structural flexibility, property tunability, and diverse functionality. Polyphosphazenes provide an opportunity to combine benefits of organic polymers and inorganic components using an inorganic backbone and organic (or organometallic) side groups. Furthermore, macromolecular substitution different organic groups and active molecules into the polyphosphazene backbone allows for control of the hydrophilic and hydrophobic balance, yielding a wide array of materials. Alongside great tunability, these materials display superior biocompatibility to typical biomaterials and can be blended with other biologically relevant polymers to yield unique erosion profiles and buffering degradation products. Researchers can capitalize on the flexibility of the backbone and multiplicity of the macromolecular substitution reaction to meet various design requirements, including stereochemistry, nanostructure, and topology. Considerable progress has been made using polyphosphazene in the fields of controlled drug/gene/vaccine delivery, regenerative engineering, cell imaging tracking, coating formulations for wound dressing, etc. This includes cyclomatrix-polyphosphazenes, which hold substantial technological prospects with respect to their synthetic design. This review provides a holistic overview of the progress of the recent three years of polyphosphazene-based materials in biomedicine.
Lay Summary
There is a pressing and economic need for new functional polymers that can be employed in numerous biomedical applications, including tissue and regenerative engineering, drug/vaccine/gene delivery, imaging technology, and medical coating formulations. Advances in materials science have enabled the introduction of novel design and synthetic approaches, which involve controlling and manipulating the biomaterial properties and performance on the molecular and atomistic levels. This controlling influence on the structure–property relationship is well prepared to meet biomedical materials’ ever-changing requirements and complexities. While several excellent polymers have emerged in this pursuit, polyphosphazene polymers present a unique avenue to expanding on an existing array of biopolymers and introducing inorganic entities with chemical versatility and neutral bioactivity. Polyphosphazene-based systems could solidify the design framework needed to diversify and personalize biomaterial to suit a wide range of biomedical needs.


Reproduced from ref. [11] with permission from the Royal Society of Chemistry



Reproduced from ref. [55] with permission from the Royal Society of Chemistry

Reproduced from ref. [30]










Similar content being viewed by others
References
Rothemund S, Teasdale I. Preparation of polyphosphazenes: a tutorial review. Chem Soc Rev. 2016;45:5200–15.
Andrianov AK, Svirkin YY, LeGolvan MP. Synthesis and biologically relevant properties of polyphosphazene polyacids. Biomacromol. 2004;5:1999–2006.
Allcock HR. Polyphosphazene elastomers, gels, and other soft materials. Soft Matter. 2012;8:7521–32.
Teasdale I, Brüggemann O. Polyphosphazenes: multifunctional, biodegradable vehicles for drug and gene delivery. Polymers. 2013;5:161–87.
Ni Z, et al. Recent research progress on polyphosphazene-based drug delivery systems. J Mater Chem B. 2020;8:1555–75.
Allcock HR, Kugel RL. Synthesis of high polymeric alkoxy- and aryloxyphosphonitriles. J Am Chem Soc. 1960;30:678.
Chen F, Teniola OR, Laurencin CT. Biodegradable polyphosphazenes for regenerative engineering. J Mater Res. 2022;37:1417–28.
Allcock HR, Morozowich NL. Bioerodible polyphosphazenes and their medical potential. Polym Chem. 2012;3:578–90.
Wan C, Huang X. Cyclomatrix polyphosphazenes frameworks (Cyclo-POPs) and the related nanomaterials: synthesis, assembly and functionalisation. Mater Today Commun. 2017;11:38–60.
Andrianov AK, Marin A, Deng J, Fuerst TR. Protein-loaded soluble and nanoparticulate formulations of ionic polyphosphazenes and their interactions on molecular and cellular levels. Mater Sci Eng C. 2020;106: 110179.
Salinas Y, et al. Dual stimuli-responsive polyphosphazene-based molecular gates for controlled drug delivery in lung cancer cells. RSC Adv. 2020;10:27305–14.
Quiñones JP, et al. Polyphosphazene-based nanocarriers for the release of agrochemicals and potential anticancer drugs. J Mater Chem B. 2019;7:7783–94.
Grinberg VY, Burova TV, Grinberg NV, Papkov VS, Khokhlov AR. Binding energetics of charged amphiphilic ligands to thermoresponsive biodegradable poly(methoxyethylaminophosphazene) hydrogels. Langmuir. 2019;35:16915–24.
Abid MA, et al. Synthesis, characterization, hydrolytic degradation, mathematical modeling and antibacterial activity of poly[bis((methoxyethoxy)ethoxy)phosphazene] (MEEP). Polym Bull. 2021;78:6059–72.
Amin AM, et al. Synthesis, characterization, hydrolytic degradation and mathematical modeling of poly[bis(2(2-methoxyethoxyethoxy diethylamino)phosphazene]. Arab J Sci Eng. 2020;45:241–7.
Zashikhina N, et al. Synthesis and characterization of macroinitiators based on polyorganophosphazenes for the ring opening polymerization of n-carboxyanhydrides. Polymers. 2021;13:1446.
Khan RU, et al. Synthesis of polyorganophosphazenes and preparation of their polymersomes for reductive/acidic dual-responsive anticancer drugs release. J Mater Sci. 2020;55:8264–84.
Khan RU, et al. Synthesis of polyorganophosphazenes and fabrication of their blend microspheres and micro/nanofibers as drug delivery systems. Int J Polym Mater Polym Biomater. 2019;69:545–66.
Wang K, Jiang L, Qiu L. Near infrared light triggered ternary synergistic cancer therapy via L-arginine-loaded nanovesicles with modification of PEGylated indocyanine green. Acta. 2021;140:506–17.
Shahzady TG, et al. Synthesis, characterization and hydrolytic degradation of p-cresol-substituted polyphosphazenes. Arab J Sci Eng. 2019;44:6445–51.
Ullah RS, et al. Synthesis of polyphosphazene and preparation of microspheres from polyphosphazene blends with PMMA for drug combination therapy. J Mater Sci. 2019;54:745–64.
Huang Y, et al. Photoluminescent biodegradable polyorganophosphazene: a promising scaffold material for in vivo application to promote bone regeneration. Bioact Mater. 2020;5:102–9.
Ogueri KS, et al. Synthesis, physicochemical analysis, and side group optimization of degradable dipeptide-based polyphosphazenes as potential regenerative biomaterials. ACS Appl Polym Mater. 2019;1:1568–78.
Huang Y, et al. Biodegradable microspheres made of conductive polyorganophosphazene showing antioxidant capacity for improved bone regeneration. Chem Eng J. 2020;397:125352.
Ni Z, et al. Polyphosphazene and non-catechol-based antibacterial injectable hydrogel for adhesion of wet tissues as wound dressing. Adv Healthc Mater. 2022;11:2101421.
Weir MD, Kaner P, Marin A, Andrianov AK. Ionic fluoropolyphosphazenes as potential adhesive agents for dental restoration applications. Regen Eng Transl Med 2021 71. 2021;7:10–20.
Tong C, et al. Hybrid polyphosphazene-organosilicon polymers as useful elastomers. ACS Appl Polym Mater. 2019;1:1881–6.
Huang Z, et al. Molecular mechanism study on effect of biodegradable amino acid ester–substituted polyphosphazenes in stimulating osteogenic differentiation. Macromol. Biosci. 2019; 19.
Huang Y, et al. Antibacterial, conductive, and osteocompatible polyorganophosphazene microscaffolds for the repair of infectious calvarial defect. J Biomed Mater Res Part A. 2021. https://doi.org/10.1002/JBM.A.37252.
Ogueri KS, Ogueri KS, Allcock HR, Laurencin CT. A regenerative polymer blend composed of glycylglycine ethyl ester-substituted polyphosphazene and poly(lactic- co -glycolic acid). ACS Appl Polym Mater. 2020;2:1169–79.
Ogueri KS, et al. In vivo evaluation of the regenerative capability of glycylglycine ethyl ester-substituted polyphosphazene and poly(lactic- co -glycolic acid) blends: a rabbit critical-sized bone defect model. ACS Biomater Sci Eng. 2021;7:1564–72.
Wu W, et al. On the understanding of dielectric elastomer and its application for all-soft artificial heart. Sci Bull. 2020;66:981–90.
Bouché M, et al. Activatable hybrid polyphosphazene-AuNP nanoprobe for ROS detection by bimodal PA/CT imaging. ACS Appl Mater Interfaces. 2019;11:28648–56.
Zhu W, et al. 131I-Labeled multifunctional polyphosphazene nanospheres for SPECT imaging-guided radiotherapy of tumors. Adv Healthc Mater. 2019;8:1901299.
Cortes MDLA, et al. Cylindrical micelles by the self-assembly of crystalline-b-coil polyphosphazene-b-P2VP block copolymers. Stabilization of Gold Nanoparticles. Molecules. 2019;24:1772.
Poscher V, Teasdale I, Salinas Y. Surfactant-free synthesis of cyclomatrix and linear organosilica phosphazene-based hybrid nanoparticles. Appl Nano Mater. 2019;2:655–60.
Aydın M, Aydın B, Sezgintürk MK. Electrochemical immunosensor for CDH22 biomarker based on benzaldehyde substituted poly(phosphazene) modified disposable ITO electrode: a new fabrication strategy for biosensors. Biosens Bioelectron. 2019;126:230–9.
Albright V, et al. Polyphosphazenes enable durable, hemocompatible, highly efficient antibacterial coatings. Biomaterials. 2020;268:120586.
Xu LC, et al. New cross-linkable poly[bis(octafluoropentoxy) phosphazene] biomaterials: synthesis, surface characterization, bacterial adhesion, and plasma coagulation responses. J Biomed Mater Res Part B Appl Biomater. 2020;108:3250–60.
Albright V, Marin A, Kaner P, Sukhishvili SA, Andrianov AK. New family of water-soluble sulfo-fluoro polyphosphazenes and their assembly within hemocompatible nanocoatings. ACS Appl Bio Mater. 2019;2:3897–906.
Andrianov AK, et al. In vivo and in vitro potency of polyphosphazene immunoadjuvants with hepatitis C virus antigen and the role of their supramolecular assembly. Cite This Mol Pharm. 2021;18:734.
Marin A, et al. Next generation polyphosphazene immunoadjuvant: synthesis, self-assembly and in vivo potency with human papillomavirus VLPs-based vaccine. Nanomedicine Nanotechnol Biol Med. 2021;33:102359.
Valencia SM, et al. Improvement of RG1-VLP vaccine performance in BALB/c mice by substitution of alhydrogel with the next generation polyphosphazene adjuvant PCEP. Hum Vaccin Immunother. 2021;17:2748–61.
Qamar B, et al. Intracellular delivery of active proteins by polyphosphazene polymers. Pharmaceutics. 2021;13:249.
Andrianov AK, et al. Supramolecular assembly of toll-like receptor 7/8 agonist into multimeric water-soluble constructs enables superior immune stimulation in vitro and in vivo. ACS Appl Bio Mater. 2020;3:3187–95.
Hsu WH, et al. Structure-optimized interpolymer polyphosphazene complexes for effective gene delivery against glioblastoma. Adv Ther. 2019;2:1800126.
Zhou N, et al. One-pot synthesis of acid-degradable polyphosphazene prodrugs for efficient tumor chemotherapy. J Mater Chem B. 2020;8:10540.
Ozay H, Ilgin P, Ozay O. Novel hydrogels based on crosslinked chitosan with formyl-phosphazene using Schiff-base reaction. Int J Polym Mater Polym Biomater. 2019;70:246–55.
Mehmood S, et al. Preparation of poly(cyclotriphosphazene-co-piperazine) nanospheres and their drug release behavior. Int J Polym Mater Polym Biomater. 2020;71:139–47.
Jing X, et al. Intelligent nanoflowers: a full tumor microenvironment-responsive multimodal cancer theranostic nanoplatform. Nanoscale. 2019;11:15508–18.
Örüm SM. Novel cyclomatrix polyphosphazene nanospheres: preparation, characterization and dual anticancer drug release application. Polym Bull. 2021;79:2851–69.
Yurtdaş-Kırımlıoğlu G, Süzen-Demircioğlu Y, Berkman MS, Metinoğlu-Örüm S, Altun E. Synthesis, spectroscopic, thermal properties, in vitro release, and stability studies of ibuprofen-loaded microspheres cross-linked with hexachlorocyclotriphosphazene/octachlorocyclotetraphosphazene. Polym Bull. 2020;78:6221–50.
MetinoğluÖrüm S, SüzenDemircioğlu Y. One-pot synthesis and characterization of crosslinked polyphosphazene dopamine microspheres for controlled drug delivery applications. J Macromol SciPart A. 2019;56:854–9.
Ozay H, Ilgin P, Ozyurt C, Ozay O. The single-step synthesis of thiol-functionalized phosphazene-based polymeric microspheres as drug carrier. Polym Technol Mater. 2020;59:1944–55.
Jing X, et al. pH/redox dual-stimuli-responsive cross-linked polyphosphazene nanoparticles for multimodal imaging-guided chemo-photodynamic therapy. Nanoscale. 2019;11:9457–67.
Wang D, et al. Facile preparation of pH/redox dual-responsive biodegradable polyphosphazene prodrugs for effective cancer chemotherapy. Colloids Surfaces B Biointerfaces. 2021;200:111573.
Onder A, Ozay H. Synthesis and characterization of biodegradable and antioxidant phosphazene-tannic acid nanospheres and their utilization as drug carrier material. Mater Sci Eng C. 2020;120:111723.
Hu X, et al. Facile synthesis of inorganic–organic hybrid fluorescent nanoparticles with AIE feature using hexachlorocyclotriphosphazene as the bridge. J Mol Liq. 2022;345:117693.
Ding G, et al. Preparation of multiple-spectra encoded polyphosphazene microspheres and application for antibody detection. Polym Bull. 2021. https://doi.org/10.1007/S00289-021-03811-W.
Guo T, et al. Rapid synthesis of amphiphilic europium complexes via ultrasonic treatment-assisted crosslinking reaction. Dye Pigment. 2022;197:109950.
Bouché M, Cormode DP. Biodegradable AuNP-based plasmonic nanogels as contrast agents for computed tomography and photoacoustics. Biomed Eng Technol. 2022;2393:773–96.
Gascón E, et al. (Amino)cyclophosphazenes as multisite ligands for the synthesis of antitumoral and antibacterial silver(I) complexes. Inorg Chem. 2020;59:2464–83.
Chelike DK, et al. Functionalized iron oxide nanoparticles conjugate of multi-anchored Schiff’s base inorganic heterocyclic pendant groups: cytotoxicity studies. Appl Surf Sci. 2020;501:143963.
Ogueri KS, Allcock HR, Laurencin CT. Generational biodegradable and regenerative polyphosphazene polymers and their blends with poly (lactic-co-glycolic acid). Prog Polym Sci. 2019;98:101146.
Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15:3640–59.
Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17:100–6.
Ge Z, et al. Histological evaluation of osteogenesis of 3D-printed poly-lactic-co-glycolic acid (PLGA) scaffolds in a rabbit model. Biomed Mater. 2009;4:021001.
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–97.
Essa D, Kondiah PPD, Choonara YE, Pillay V. The design of poly(lactide-co-glycolide) nanocarriers for medical applications. Front Bioeng Biotechnol. 2020;8:48.
Echeverria Molina MI, Malollari KG, Komvopoulos K. Design challenges in polymeric scaffolds for tissue engineering. Front Bioeng Biotechnol. 2021;9:617141.
Bu Y, Ma J, Bei J, Wang S. Surface modification of aliphatic polyester to enhance biocompatibility. Front Bioeng Biotechnol. 2019;0:98.
Piotrowska U, Sobczak M, Oledzka E. Characterization of aliphatic polyesters synthesized via enzymatic ring-opening polymerization in ionic liquids. Molecules. 2017;22:923.
Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci Part B Polym Phys. 2011;49:832–64.
Mouthuy PA, et al. Biocompatibility of implantable materials: an oxidative stress viewpoint. Biomaterials. 2016;109:55–68.
Wang L, et al. Visual in vivo degradation of injectable hydrogel by real-time and non-invasive tracking using carbon nanodots as fluorescent indicator. Biomaterials. 2017;145:192–206.
Ahmad D, Patra K, Hossain M. Experimental study and phenomenological modelling of flaw sensitivity of two polymers used as dielectric elastomers. Contin Mech Thermodyn. 2020;32:489–500.
Brochu P, Pei Q. Dielectric elastomers for actuators and artificial muscles. Electroact Polym Mater. 2012. https://doi.org/10.1007/978-1-4614-0878-9_1.
Andrianov AK, Langer R. Polyphosphazene immunoadjuvants: historical perspective and recent advances. J Control Release. 2021;329:299–315.
Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012;28:87–101.
Albright V, et al. Fluorinated polyphosphazene coatings using aqueous nano-assembly of polyphosphazene polyelectrolytes. ACS Symp Ser. 2018;1298:101–18.
Selin V, et al. Biocompatible nanocoatings of fluorinated polyphosphazenes through aqueous assembly. ACS Appl Mater Interfaces. 2018;10:9756–64.
Cheng Y, Feng G, Moraru CI. Micro-and nanotopography sensitive bacterial attachment mechanisms: a review. Front Microbiol. 2019;10:191.
Quan WY, et al. Mussel-inspired catechol-functionalized hydrogels and their medical applications. Molecules. 2019;24:2586.
Aied A, Greiser U, Pandit A, Wang W. Polymer gene delivery: overcoming the obstacles. Drug Discov Today. 2013;18:1090–8.
Otsuka T, Kan H-M, Laurencin CT. Regenerative engineering approaches to scar-free skin regeneration. Regen Eng Transl Med. 2022;8(2):225–47. https://doi.org/10.1007/s40883-021-00229-8.
Ude CC, Shah S, Ogueri KS, Nair LS, Laurencin CT. Stromal vascular fraction for osteoarthritis of the knee regenerative engineering. Regen Eng Transl Med. 2022;8(2):210–24. https://doi.org/10.1007/s40883-021-00226-x.
Mengsteab PY, Freeman J, Barajaa MA, Nair LS, Laurencin CT. Ligament regenerative engineering: braiding scalable and tunable bioengineered ligaments using a bench-top braiding machine. Regen Eng Transl Med. 2021;7(4):524–32. https://doi.org/10.1007/s40883-020-00178-8.
Beachy SH, Nair L, Laurencin C, Tsokas KA, Lundberg MS. Sources of variability in clinical translation of regenerative engineering products: insights from the national academies forum on regenerative medicine. Regen Eng Transl Med. 2020;6(1):1–6. https://doi.org/10.1007/s40883-020-00151-5.
Tang X, Daneshmandi L, Awale G, Nair LS, Laurencin CT. Skeletal muscle regenerative engineering. Regen Eng Transl Med. 2019;5(3):233–51. https://doi.org/10.1007/s40883-019-00102-9.
Tang X, Saveh-Shemshaki N, Kan H-M, Khan Y, Laurencin CT. Biomimetic electroconductive nanofibrous matrices for skeletal muscle regenerative engineering. Regen Eng Transl Med. 2020;6(2):228–37. https://doi.org/10.1007/s40883-019-00136-z.
Nelson C, Khan Y, Laurencin CT. Nanofiber/microsphere hybrid matrices in vivo for bone regenerative engineering: a preliminary report. Regen Eng Transl Med. 2018;4(3):133–41. https://doi.org/10.1007/s40883-018-0055-1.
Ogueri KS, Ivirico JLE, Nair LS, Allcock HR, Laurencin CT. Biodegradable polyphosphazene-based blends for regenerative engineering. Regen Eng Transl Med. 2017;3(1):15–31. https://doi.org/10.1007/s40883-016-0022-7.
Narayanan N, Jiang C, Uzunalli G, Thankappan SK, Laurencin CT, Deng M. Polymeric electrospinning for musculoskeletal regenerative engineering. Regen Eng Transl Med. 2016;2(2):69–84. https://doi.org/10.1007/s40883-016-0013-8.
Kasir R, Vernekar VN, Laurencin CT. Regenerative engineering of cartilage using adipose-derived stem cells. Regen Eng Transl Med. 2015;1(1–4):42–9. https://doi.org/10.1007/s40883-015-0005-0.
Fatemeh S, Hosseini Cato T. Laurencin advanced electrospun nanofibrous stem cell niche for bone regenerative engineering. Regen Eng Transl Med. https://doi.org/10.1007/s40883-022-00274-x.
Esdaille CJ, Ude CC, Laurencin CT. Regenerative engineering animal models for knee osteoarthritis. Regen Eng Transl Med. 2022;8(2):284–97. https://doi.org/10.1007/s40883-021-00225-y.
Benton Swanson W, Mishina Y. New paradigms in regenerative engineering: emerging role of extracellular vesicles paired with instructive biomaterials. Biocell. 2022;46(6):1445–51. https://doi.org/10.32604/biocell.2022.018781.
Zhuge W, Liu H, Wang W, Wang J. Microfluidic bioscaffolds for regenerative engineering. Engineered Regeneration. 2022;3(1):110–20. https://doi.org/10.1016/j.engreg.2021.12.003.
Ryan H, Bister D, Holliday SA, Boehlein J, Lewis A, Silberman J, Allen JB, Moore E. Ancestral background is underreported in regenerative engineering. Regen Eng Transl Med. 2021. https://doi.org/10.1007/s40883-021-00237-8.
Ameer GA. Understanding and harnessing variability in regenerative engineering. Regen Eng Transl Med. 2020;6(4):429–32. https://doi.org/10.1007/s40883-020-00155-1.
Tao F, Cheng Y, Shi X, Zheng H, Du Y, Xiang W, Deng H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr Polym. 2020;230:115658. https://doi.org/10.1016/j.carbpol.2019.115658.
Wang X, Rivera-Bolanos N, Jiang B, Ameer GA. Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine. Adv Funct Mater. 2019;29(23):1809009. https://doi.org/10.1002/adfm.201809009.
Heath DE. A review of decellularized extracellular matrix biomaterials for regenerative engineering applications. Regen Eng Transl Med. 2019;5(2):155–66. https://doi.org/10.1007/s40883-018-0080-0.
Ma C, Kuzma ML, Bai X, Yang J. Biomaterial-based metabolic regulation in regenerative engineering. Adv Sci. 2019;6(19):1900819. https://doi.org/10.1002/advs.201900819.
Ong J, Zhao J, Justin AW, Markaki AE. Albumin‐based hydrogels for regenerative engineering and cell transplantation. Biotechnol Bioeng. 2019;116(12):3457–68. https://doi.org/10.1002/bit.27167.
Moore EM, West JL. Bioactive poly(ethylene glycol) acrylate hydrogels for regenerative engineering. Regen Eng Transl Med. 2019;5(2):167–79. https://doi.org/10.1007/s40883-018-0074-y.
Bowers DT, Brown JL. Nanofibers as bioinstructive scaffolds capable of modulating differentiation through mechanosensitive pathways for regenerative engineering. Regen Eng Transl Med. 2019;5(1):22–9. https://doi.org/10.1007/s40883-018-0076-9.
Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Weikel AL, Krogman NR, Allcock HR, Laurencin CT. In situ porous structures: a unique polymer erosion mechanism in biodegradable dipeptide-based polyphosphazene and polyester blends producing matrices for regenerative engineering. Adv Funct Mater. 2010;20(17):2794–806. https://doi.org/10.1002/adfm.201000968.
Lo KW-H, Ashe KM, Kan HM, Lee DA, Laurencin CT. Activation of cyclic amp/protein kinase: a signaling pathway enhances osteoblast cell adhesion on biomaterials for regenerative engineering. J Orthop Res. 2011;29(4):602–8. https://doi.org/10.1002/jor.21276.
Deng M, Cushnie EK, Lv Q, Laurencin CT. Poly(lactide-co-glycolide)-hydroxyapatite composites: the development of osteoinductive scaffolds for bone regenerative engineering. MRS Proc. 2012;1417:737. https://doi.org/10.1557/opl.2012.737.
Deng M, James R, Laurencin CT, Kumbar SG. Nanostructured polymeric scaffolds for orthopaedic regenerative engineering. IEEE Trans Nanobioscience. 2012;11(1):3–14. https://doi.org/10.1109/TNB.2011.2179554.
Lo KW-H, Ulery BD, Kan HM, Ashe KM, Laurencin CT. Evaluating the feasibility of utilizing the small molecule phenamil as a novel biofactor for bone regenerative engineering. J Tissue Eng Regen Med. 2014;8(9):728–36. https://doi.org/10.1002/term.1573.
Laurencin CT, Khan Y. Regenerative engineering. Sci Transl Med. 2012;4(160):160ed9. https://doi.org/10.1126/scitranslmed.3004467.
McLaughlin SW, Nelson SJ, McLaughlin WM, Nair LS, Laurencin CT. Design of nanofibrous scaffolds for skeletal muscle regenerative engineering. J Biomater Tissue Eng. 2013;3(4):385–95. https://doi.org/10.1166/jbt.2013.1107.
Lo KW-H, Jiang T, Gagnon KA, Nelson C, Laurencin CT. Small-molecule based musculoskeletal regenerative engineering. Trends Biotechnol. 2014;32(2):74–81. https://doi.org/10.1016/j.tibtech.2013.12.002.
Mikael PE, Amini AR, Basu J, Josefina Arellano-Jimenez M, Laurencin CT, Sanders MM, Barry Carter C, Nukavarapu SP. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: fabrication, in vitro and in vivo evaluation. Biomed Mater. 2014;9(3):035001. https://doi.org/10.1088/1748-6041/9/3/035001.
Jiang T, Deng M, James R, Nair LS, Laurencin CT. Micro- and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014;10(4):1632–45. https://doi.org/10.1016/j.actbio.2013.07.003.
Shelke NB, James R, Laurencin CT, Kumbar SG. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym Adv Technol. 2014;25(5):448–60. https://doi.org/10.1002/pat.3266.
Laurencin CT, Ashe KM, Henry N, Kan HM, Lo KW-H. Delivery of small molecules for bone regenerative engineering: preclinical studies and potential clinical applications. Drug Discov Today. 2014;19(6):794–800. https://doi.org/10.1016/j.drudis.2014.01.012.
Cushnie EK, Ulery BD, Nelson SJ, Deng M, Sethuraman S, Doty SB, Lo KWH, Khan YM, Laurencin CT, Chin W-C. Simple signaling molecules for inductive bone regenerative engineering. PLoS ONE. 2014;9(7):e101627. https://doi.org/10.1371/journal.pone.0101627.
Laurencin CT, James R. Composites and structures for regenerative engineering. MRS Proceedings. 2014;1621:3–15. https://doi.org/10.1557/opl.2014.4.
Nelson C, Khan Y, Laurencin CT. Nanofiber-microsphere (nano-micro) matrices for bone regenerative engineering: a convergence approach toward matrix design. Regenerative Biomaterials. 2014;1(1):3–9. https://doi.org/10.1093/rb/rbu002.
James R, Harmon MD, Kumbar SG, Laurencin CT. Innovative regenerative engineering technologies for soft tissue regeneration. Technol Innov. 2014;16(3):195–214. https://doi.org/10.3727/194982414X14138187301579.
Laurencin C, Jiang T, Kumbar S, Nair L. Biologically active chitosan systems for tissue engineering and regenerative medicine. Curr Top Med Chem. 2008;8(4):354–64. https://doi.org/10.2174/156802608783790974.
Yu X, Tang X, Gohil SV, Laurencin CT. Biomaterials for bone regenerative engineering. Adv Healthc Mater. 2015;4(9):1268–85. https://doi.org/10.1002/adhm.201400760.
James R, Mengsteab P, Laurencin CT. Regenerative engineering: studies of the rotator cuff and other musculoskeletal soft tissues. MRS Advances. 2016;1(18):1255–63. https://doi.org/10.1557/adv.2016.282.
James R, Laurencin CT. Regenerative engineering and bionic limbs. Rare Metals. 2015;34(3):143–55. https://doi.org/10.1007/s12598-015-0446-0.
Laurencin CT, Nair LS. Regenerative engineering: approaches to limb regeneration and other grand challenges. Regen Eng Transl Med. 2015;1(1–4):1–3. https://doi.org/10.1007/s40883-015-0006-z.
Vernekar VN, James R, Smith KJ, Laurencin CT. Nanotechnology applications in stem cell science for regenerative engineering. J Nanosci Nanotechnol. 2016;16(9):8953–65. https://doi.org/10.1166/jnn.2016.12738.
Narayanan G, Vernekar VN, Kuyinu EL, Laurencin CT. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Deliv Rev. 2016;107:247–76. https://doi.org/10.1016/j.addr.2016.04.015.
Laurencin CT., Nair LS. The Quest toward limb regeneration: a regenerative engineering approach. Regen Biomater. 2016;3(2):123–125. https://doi.org/10.1093/rb/rbw002.
Mengsteab PY, Nair LS, Laurencin CT. The past, present and future of ligament regenerative engineering. Regen Med. 2016;11(8):871–81. https://doi.org/10.2217/rme-2016-0125.
Xiaoyan T, Yusuf K, Cato L. Electroconductive nanofiber scaffolds for muscle regenerative engineering. Front Bioeng Biotechnol. 2016;4. https://doi.org/10.3389/conf.FBIOE.2016.01.02165.
Escobar Ivirico JL, Bhattacharjee M, Kuyinu E, Nair LS, Laurencin CT. Regenerative engineering for knee osteoarthritis treatment: biomaterials and cell-based technologies. Engineering. 2017;3(1):16–27. https://doi.org/10.1016/J.ENG.2017.01.003.
Narayanan G, Bhattacharjee M, Nair LS, Laurencin CT. Musculoskeletal tissue regeneration: the role of the stem cells. Regen Eng Transl Med. 2017;3(3):133–65. https://doi.org/10.1007/s40883-017-0036-9.
Ifegwu OC, Awale G, Rajpura K, Lo KW-H, Laurencin CT. Harnessing cAMP signaling in musculoskeletal regenerative engineering. Drug Discov Today. 2017;22(7):1027–44. https://doi.org/10.1016/j.drudis.2017.03.008.
Narayanan G, Nair LS, Laurencin CT. Regenerative engineering of the rotator cuff of the shoulder. ACS Biomater Sci Eng. 2018;4(3):751–86. https://doi.org/10.1021/acsbiomaterials.7b00631.
Peach MS, Ramos DM, James R, Morozowich NL, Mazzocca AD, Doty SB, Allcock HR, Kumbar SG, Laurencin CT, Engler AJ. Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLOS ONE. 2017;12(4):e0174789. https://doi.org/10.1371/journal.pone.0174789.
Ogueri KS, Jafari T, Escobar Ivirico JL, Laurencin CT. Polymeric biomaterials for scaffold-based bone regenerative engineering. Regen Eng Transl Med. 2019;5(2):128–54. https://doi.org/10.1007/s40883-018-0072-0.
Saveh-Shemshaki N, Nair LS, Laurencin CT. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater. 2019;94:64–81. https://doi.org/10.1016/j.actbio.2019.05.041.
Barajaa MA, Nair LS, Laurencin CT. Bioinspired scaffold designs for regenerating musculoskeletal tissue interfaces. Regen Eng Transl Med. 2020;6(4):451–83. https://doi.org/10.1007/s40883-019-00132-3.
Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. https://doi.org/10.1016/j.biomaterials.2019.119536.
Laurencin CT, McClinton A. Regenerative cell-based therapies: cutting edge, bleeding edge, and off the edge. Regen Eng Transl Med. 2020;6(1):78–89. https://doi.org/10.1007/s40883-020-00147-1.
Montgomery AB, McClinton A, Nair L, Laurencin CT. Nail matrix regenerative engineering: in vitro evaluation of poly(lactide-co-glycolide)/gelatin fibrous substrates. J Biomed Mater Res A. 2020;108(5):1136–43. https://doi.org/10.1002/jbm.a.36888.
Ogueri KS, Ogueri KS, Allcock HR, Laurencin CT. Polyphosphazene polymers: the next generation of biomaterials for regenerative engineering and therapeutic drug delivery. J Vac Sci Technol B. 2020;38(3):030801. https://doi.org/10.1116/6.0000055.
Laurencin CT, Daneshmandi L. Graphene for regenerative engineering. Int J Appl Ceram Eng Sci. 2020;2(3):140–3. https://doi.org/10.1002/ces2.10045.
Daneshmandi L, Shah S, Jafari T, Bhattacharjee M, Momah D, Saveh-Shemshaki N, Lo KW-H, Laurencin CT. Emergence of the stem cell secretome in regenerative engineering. Trends Biotechnol. 2020;38(12):1373–84. https://doi.org/10.1016/j.tibtech.2020.04.013.
Daneshmandi L, Barajaa M, Tahmasbi Rad A, Sydlik SA, Laurencin CT. Graphene‐based biomaterials for bone regenerative engineering: a comprehensive review of the field and considerations regarding biocompatibility and biodegradation. Adv Healthc Mater. 2021;10(1):2001414. https://doi.org/10.1002/adhm.202001414.
Ude CC, Esdaille CJ, Ogueri KS, Kan H-M, Laurencin SJ, Nair LS, Laurencin CT. The mechanism of metallosis after total hip arthroplasty. Regen Eng Transl Med. 2021;7(3):247–61. https://doi.org/10.1007/s40883-021-00222-1.
Hosseini FS, Nair LS, Laurencin CT. Inductive materials for regenerative engineering. J Dent Res. 2021;100(10):1011–9. https://doi.org/10.1177/00220345211010436.
Prabhath A, Vernekar VN, Vasu V, Badon M, Avochinou J‐E, Asandei AD, Kumbar SG, Weber E, Laurencin CT. Kinetic degradation and biocompatibility evaluation of polycaprolactone-based biologics delivery matrices for regenerative engineering of the rotator cuff. J Biomed Mater Res A. 2021;109(11):2137–53. https://doi.org/10.1002/jbm.a.37200.
Washington KS, Shemshaki NS, Laurencin CT. The role of nanomaterials and biological agents on rotator cuff regeneration. Regen Eng Transl Med. 2021;7(4):440–9. https://doi.org/10.1007/s40883-020-00171-1.
Ude CC, Shah S, Ogueri KS, Nair LS, Laurencin CT. Stromal vascular fraction for osteoarthritis of the knee regenerative engineering. Regen Eng Transl Med. 2022;8(2):210–24. https://doi.org/10.1007/s40883-021-00226-x.
Hosseini FS, Laurencin CT. Advanced graphene ceramics and their future in bone regenerative engineering. Int J Appl Ceram Technol. 2022;19(2):893–905. https://doi.org/10.1111/ijac.13999.
Shah S, Esdaille CJ, Bhattacharjee M, Kan H-M, Laurencin CT. The synthetic artificial stem cell (SASC): shifting the paradigm of cell therapy in regenerative engineering. Proc Natl Acad Sci. 2022;119(2):e2116865118. https://doi.org/10.1073/pnas.2116865118.
Funding
The study was financially supported by the NIH 1T32AR079114-01 and the Connecticut Convergence Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, F., Teniola, O.R., Ogueri, K.S. et al. Recent Trends in the Development of Polyphosphazenes for Bio-applications. Regen. Eng. Transl. Med. 9, 202–223 (2023). https://doi.org/10.1007/s40883-022-00278-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-022-00278-7