Abstract
Bioreactors have immense value within tissue engineering by enabling important control over design inputs within a microenvironment; however, they are often complex and expensive. Herein, we demonstrate a strategy where a bioreactor was 3D-printed as a means to provide an accessible route to culture scaffolds under physiological inputs and to determine an ideal tendon scaffold structure based on remodeling and degradation events. Furthermore, real-time monitoring of tissue remodeling is introduced through the bioreactor design. Following in vitro uniaxial stretch conditioning, scaffolds were positively altered to reflect improved biomechanical function, enhanced extracellular matrix (ECM) content, and increased mechanical properties of elastic moduli. A bioreactor was constructed to meet the following criteria: real-time readout of resistance, ability to be easily cleaned and sterilized, control of strain, speed, and time parameters, and capability to withstand 3 weeks of continuous load with minimal maintenance. The scaffolds cultured in a dynamic setting showed improved mechanics and promising ECM and collagen content generated. Overall, the bioreactor was effective as a tool to provide mechanical culture conditions that promote the tendon cell niche. The bioreactor supports the scaffolds as a possible therapeutic venture in tendon tissue engineering. Additionally, the 3D-printed bioreactor is a significant contribution to the tissue engineering field by making dynamic culturing more accessible for researchers.
Lay Summary
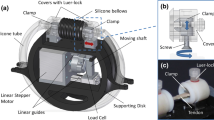
Bioreactors present physiological and dynamic conditions for tissue testing and are crucial in tissue engineering for product development, standardization, quality control, and large-scale synthesis. The low-cost, 3D-printed, programmable bioreactor provides cyclic stretch to scaffolds with real-time resistance readout and demonstrates tissue changes over time. The bioreactor enhances scaffold biomechanics and biofunctionality towards natural tendon by promoting cell alignment through mechanical force. The design components are culture chamber for tendon constructs, motor control of uniaxial stretching, and a base for stabilization. The results from conditioning scaffolds in the bioreactor lend to a better understanding of how specific inputs affect tissue outcomes.





Similar content being viewed by others
References
Youngstrom DW, Barrett JG. Engineering tendon: scaffolds, bioreactors, and models of regeneration. Stem Cells Int. 2016;2015:1–11.
Matheson LA, Fairbank NJ, Maksym GN, Santerre JP, Labow RS. Characterization of the Flexcell (TM) Uniflex (TM) cyclic strain culture system with U937 macrophage-like cells. Biomaterials. 2006;27:226–33.
Youngstrom DW, Rajpar I, Kaplan DL, Barrett JG. A bioreactor system for in vitro tendon differentiation and tendon tissue engineering. Andarawis-Puri N, Flatow EL, Soslowsky LJ, editors. J Orthop Res. 2015;33:911–8.
Wang T, Lin Z, Day RE, Gardiner B, Landao-Bassonga E, Rubenson J, et al. Programmable mechanical stimulation influences tendon homeostasis in a bioreactor system. Biotechnol Bioeng. 2013;110:1495–507.
Wang T, Lin Z, Ni M, Thien C, Day RE, Gardiner B, et al. Cyclic mechanical stimulation rescues achilles tendon from degeneration in a bioreactor system. J Orthop Res. 2015;33:1888–96.
Maeda E, Hagiwara Y, Wang JH-C, Ohashi T. A new experimental system for simultaneous application of cyclic tensile strain and fluid shear stress to tenocytes in vitro. Biomed Microdevices. 2013;15:1067–75.
Plunkett N, O’Brien FJ. Bioreactors in tissue engineering. Technol Health Care. 2011;19:55–69.
Zhao J, Griffin M, Cai J, Li S, Butler P, Kalaskar DM. Bioreactors for tissue engineering: an update. Biochem Eng J. 2016;109:268–81.
Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker’s guide to mechanobiology. Dev Cell. 2011;21:35–47.
Chaturvedi LS, Marsh HM, Basson MD. Src and focal adhesion kinase mediate mechanical strain-induced proliferation and ERK1/2 phosphorylation in human H441 pulmonary epithelial cells. Am J Physiol Cell Physiol. 2007;292:C1701–13.
Driscoll TP, Cosgrove BD, Heo S-J, Shurden ZE, Mauck RL. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys J. 2015;108:2783–93.
Boutahar N, Guignandon A, Vico L, Lafage-Proust M. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279:30588–99.
Hong S-Y, Jeon Y-M, Lee H-J, Kim J-G, Baek J-A, Lee J-C. Activation of RhoA and FAK induces ERK-mediated osteopontin expression in mechanical force-subjected periodontal ligament fibroblasts. Mol Cell Biochem. 2010;335:263–72.
Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26.
Higgins AM, Banik BL, Brown JL. Geometry sensing through POR1 regulates Rac1 activity controlling early osteoblast differentiation in response to nanofiber diameter. Integr Biol. 2015;7:229–36.
Ozdemir T, Xu L-C, Siedlecki C, Brown JL. Substrate curvature sensing through Myosin IIa upregulates early osteogenesis. Integr Biol. 2013;5:1407–16.
Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28:564–72.
Kim H, Sonn JK. Rac1 promotes chondrogenesis by regulating STAT3 signaling pathway. Cell Biol Int. 2016;40:976–83.
Woods A, Beier F. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem. 2006;281:13134–40.
McBeath R, Fertala A, Risbud M, Shapiro I, Osterman L. Hypoxia drives tenocyte differentiation. J Hand Surg. 2013;38:e11.
Banik BL, Lewis GS, Brown JL. Multiscale poly-(ϵ-caprolactone) scaffold mimicking non-linearity in tendon tissue mechanics. Regen Eng Transl Med. 2016;2:1–9.
Wang T, Gardiner BS, Lin Z, Rubenson J, Kirk TB, Wang A, et al. Bioreactor design for tendon/ligament engineering. Tissue Eng B. 2012;19:133–46.
Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 22:80–6.
Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg. 2005;87:187–202.
Nirmalanandhan VS, Dressler MR, Shearn JT, Juncosa-Melvin N, Rao M, Gooch C, et al. Mechanical stimulation of tissue engineered tendon constructs: effect of scaffold materials. J Biomech Eng. 2007;129:919–23.
Angelidis IK, Thorfinn J, Connolly ID, Lindsey D, Pham HM, Chang J. Tissue engineering of flexor tendons: the effect of a tissue bioreactor on adipoderived stem cell–seeded and fibroblast-seeded tendon constructs. J Hand Surg. 2010;35A:1466–72.
Saber S, Zhang AY, Ki SH, Lindsey DP, Smith RL, Riboh J, et al. Flexor tendon tissue engineering: bioreactor cyclic strain increases construct strength. Tissue Eng A. 2010;16:2085–90.
McMahon JT, Myles JL, Tubbs RR. Demonstration of immune complex deposits using fluorescence microscopy of hematoxylin and eosin–stained sections of Hollande’s fixed renal biopsies. Mod Pathol. 2002;15:988–97.
Heo YS, Song HJ. Characterizing cutaneous elastic fibers by eosin fluorescence detected by fluorescence microscopy. Ann Dermatol. 2011;23:44–52.
Elston DM. Medical Pearl: Fluorescence microscopy of hematoxylin-eosin-stained sections. J Am Acad Dermatol. 2002;47:777–9.
Deeb S, Nesr KH, Mahdy E, Badawey M, Badei M. Autofluorescence of routinely hematoxylin and eosinstained sections without exogenous markers. Afr J Biotechnol. 7:504–7.
Acknowledgments
Gene Gerber, Tony Banik, Andrew Higgins, Jordan Dover, Gary Meyers, Austin Greever, Greg Learn, Eric VanArsdale, Michele Herneisey, and Nimesh Lad are acknowledged for their input on the bioreactor design and iterations. John Cantolina is recognized for his help with histology.
Funding
This material was based upon work supported by R03 AR065192 and the National Science Foundation (NSF) under Grant No. DGE1255832. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Description of Future Works
Future works to expand this project include the following: integrating a Graphic User Interface (GUI) for the bioreactor to simplify programming, testing silk as a scaffold material, and consideration to overall scaffold design that would incorporate the enthesis.
Electronic supplementary material
ESM 1
(ZIP 171 kb)
Rights and permissions
About this article
Cite this article
Banik, B.L., Brown, J.L. 3D-Printed Bioreactor Enhances Potential for Tendon Tissue Engineering. Regen. Eng. Transl. Med. 6, 419–428 (2020). https://doi.org/10.1007/s40883-019-00145-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00145-y