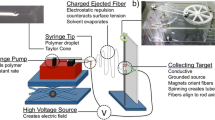
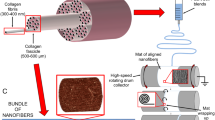
Abstract
Tissue engineering has showed promising results in restoring diseased tendon tissue functions. Herein, a hybrid three-dimensional (3D) porous scaffold comprising an outer portion rolled from an electrohydrodynamic jet printed poly(ɛ-caprolactone) (PCL) fiber mesh, and an inner portion fabricated from uniaxial stretching of a heat-sealed PCL tube, was developed for tendon tissue engineering (TE) application. The outer portion included three layers of micrometer-scale fibrous bundles (fiber diameter: ~25 µm), with an interconnected spacing and geometric anisotropy along the scaffold length. The inner portion showed orientated micro-ridges/grooves in a parallel direction to that of the outer portion. Owning to the addition of the inner portion, the as-fabricated scaffold exhibited comparable mechanical properties to those of the human patellar tendon in terms of Young’s modulus (~227 MPa) and ultimate tensile stress (~50 MPa). Compared to the rolled electrospun fibers, human tenocytes cultured in the tendon scaffolds showed increased cellular metabolism. Furthermore, the 3D tendon scaffold resulted in up-regulated cell alignment, cell elongation and formation of collagen type I. These results demonstrated the potential of mechanically-enhanced 3D fibrous scaffold for applications in tendon TE, with desired cell alignment and functional differentiation.








Similar content being viewed by others
References
Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, et al. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng. 2006;12(2):369–79.
Lomas A, Ryan C, Sorushanova A, Shologu N, Sideri A, Tsioli V, et al. The past, present and future in scaffold-based tendon treatments. Adv Drug Deliv Rev. 2015;84:257–77.
Goh JCH, Ouyang HW, Teoh SH, Chan CK, Lee EH. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng. 2003;9(4 Supplement 1):31–44.
Chen X, Qi YY, Wang LL, Yin Z, Yin GL, Zou XH, et al. Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials. 2008;29(27):3683–92.
Fan H, Liu H, Toh SL, Goh JCH. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30(28):4967–77.
Cooper JA Jr, Sahota JS, Gorum WJ 2nd, Carter J, Doty SB, Laurencin CT. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci USA. 2007;104(9):3049–54.
Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng. 2008;15(1):115–26.
Xie J, Li X, Lipner J, Manning CN, Schwartz AG, Thomopoulos S, et al. “Aligned-to-random” nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site. Nanoscale. 2010;2(6):923–6.
Barber JG, Handorf AM, Allee TJ, Li WJ. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng Part A. 2011;19(11–12):1265–74.
Lu HH, Cooper JA Jr, Manuel S, Freeman JW, Attawia MA, Ko FK, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26(23):4805–16.
Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D. Fiber-based tissue engineering: progress, challenges, and opportunities. Biotechnol Adv. 2013;31(5):669–87.
Sahoo S, Ouyang H, Goh JC, Tay TE, Toh SL. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 2006;12(1):91–9.
Enea D, Gwynne J, Kew S, Arumugam M, Shepherd J, Brooks R, et al. Collagen fibre implant for tendon and ligament biological augmentation. In vivo study in an ovine model. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1783–93.
Zeugolis D, Paul R, Attenburrow G. Extruded collagen-polyethylene glycol fibers for tissue engineering applications. J Biomed Mater Res B. 2008;85(2):343–52.
Kew SJ, Gwynne JH, Enea D, Brookes R, Rushton N, Best SM, et al. Synthetic collagen fascicles for the regeneration of tendon tissue. Acta Biomater. 2012;8(10):3723–31.
Wu Y, Wang Z, Fuh JYH, Wong YS, Wang W, Thian ES. Direct E-jet printing of three-dimensional fibrous scaffold for tendon tissue engineering. J Biomed Mater Res B. 2015;. doi:10.1002/jbm.b.33580.
Wu Y, Fuh JYH, Wong YS, Sun J. Fabrication of 3D scaffolds via E-jet printing for tendon tissue repair. ASME 2015 international manufacturing science and engineering conference. 2015;2:V002T03A5.
Wang ZY, Teoh SH, Johana NB, Chong MSK, Teo EY, Hong MH, et al. Enhancing mesenchymal stem cell response using uniaxially stretched poly (ε-caprolactone) film micropatterns for vascular tissue engineering application. J Mater Chem B. 2014;2(35):5898–909.
Averous L, Moro L, Dole P, Fringant C. Properties of thermoplastic blends: starch-polycaprolactone. Polymer. 2000;41(11):4157–67.
Thomas V, Jose MV, Chowdhury S, Sullivan JF, Dean DR, Vohra YK. Mechano-morphological studies of aligned nanofibrous scaffolds of polycaprolactone fabricated by electrospinning. J Biomater Sci Polym Ed. 2006;17(9):969–84.
Tiaw KS, Teoh SH, Chen R, Hong MH. Processing methods of ultrathin poly (ε-caprolactone) films for tissue engineering applications. Biomacromolecules. 2007;8(3):807–16.
Wang ZY, Teo EY, Chong MS, Zhang QY, Lim J, Zhang ZY, et al. Biomimetic three-dimensional anisotropic geometries by uniaxial stretch of poly(ε-caprolactone) films for mesenchymal stem cell proliferation, alignment, and myogenic differentiation. Tissue Eng Part C. 2013;19(7):538–49.
Cheng Z, Teoh S-H. Surface modification of ultra thin poly (ε-caprolactone) films using acrylic acid and collagen. Biomaterials. 2004;25(11):1991–2001.
Woodruff MA, Hutmacher DW. The return of a forgotten polymer-polycaprolactone in the 21st century. Prog Polym Sci. 2010;35(10):1217–56.
Serrano M-C, Pagani R, Vallet-Regí M, Peña J, Comas J-V, Portolés M-T. Nitric oxide production by endothelial cells derived from blood progenitors cultured on NaOH-treated polycaprolactone films: a biofunctionality study. Acta Biomater. 2009;5(6):2045–53.
Wang ZY, Lim J, Ho YS, Zhang QY, Chong MS, Tang M, et al. Biomimetic three-dimensional anisotropic geometries by uniaxial stretching of poly (ε-caprolactone) films: degradation and mesenchymal stem cell responses. J Biomed Mater Res A. 2014;102(7):2197–207.
Yeong WY, Yu H, Lim KP, Ng KL, Boey YC, Subbu VS, et al. Multiscale topological guidance for cell alignment via direct laser writing on biodegradable polymer. Tissue Eng Part C. 2010;16(5):1011–21.
Stäubli HU, Schatzmann L, Brunner P, Rincón L, Nolte LP. Mechanical tensile properties of the quadriceps tendon and patellar ligament in young adults. Am J Sports Med. 1999;27(1):27–34.
Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res. 1994;12(6):796–803.
Ralphs JR, Waggett AD, Benjamin M. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002;21(1):67–74.
McNamara LE, McMurray RJ, Biggs MJP, Kantawong F, Oreffo ROC, Dalby MJ. Nanotopographical control of stem cell differentiation. J Tissue Eng. 2010;1(1):120623.
Noyes FR, Grood ES. The strength of the anterior cruciate ligament in humans and Rhesus. J Bone Joint Surg Am. 1976;58:1074–82.
Dutta RC, Dutta AK. Cell-interactive 3D-scaffold; advances and applications. Biotechnol Adv. 2009;27(4):334–9.
Sands RW, Mooney DJ. Polymers to direct cell fate by controlling the microenvironment. Curr Opin Biotechnol. 2007;18(5):448–53.
Acknowledgments
This work was supported by A*STAR Science and Engineering Research Council, Singapore under the Public Sector Funding 2012 Project Number 132 120 2074. Also, thanks for awarding a National University of Singapore Research Scholarship to YW.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Y., Wang, Z., Fuh, J.Y.H. et al. Mechanically-enhanced three-dimensional scaffold with anisotropic morphology for tendon regeneration. J Mater Sci: Mater Med 27, 115 (2016). https://doi.org/10.1007/s10856-016-5728-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5728-z