Abstract
Background
Glomerulonephritis inherently leads to the development of chronic kidney disease. It is the second most common diagnosis in patients requiring renal replacement therapy in the United Kingdom. Metabolomics and proteomics can characterise, identify and quantify an individual’s protein and metabolite make-up. These techniques have been optimised and can be performed on samples including kidney tissue, blood and urine. Utilising omic techniques in nephrology can uncover disease pathophysiology and transform the diagnostics and treatment options for glomerulonephritis.
Objectives
To evaluate the utility of metabolomics and proteomics using mass spectrometry and nuclear magnetic resonance in glomerulonephritis.
Methods
The systematic review was registered on PROSPERO (CRD42023442092). Standard and extensive Cochrane search methods were used. The latest search date was March 2023. Participants were of any age with a histological diagnosis of glomerulonephritis. Descriptive analysis was performed, and data presented in tabular form. An area under the curve or p-value was presented for potential biomarkers discovered.
Results
Twenty-seven studies were included (metabolomics (n = 9)), and (proteomics (n = 18)) with 1818 participants. The samples analysed were urine (n = 19) blood (n = 4) and biopsy (n = 6). The typical outcome themes were potential biomarkers, disease phenotype, risk of progression and treatment response.
Conclusion
This review shows the potential of metabolomic and proteomic analysis to discover new disease biomarkers that may influence diagnostics and disease management. Further larger-scale research is required to establish the validity of the study outcomes, including the several proposed biomarkers.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney disease is increasingly becoming a significant worldwide health burden [1]. The global all-age chronic kidney disease (CKD) mortality increased by 41.5% between 1990 and 2019 [2, 3]. There is a growing, unified acknowledgement of an unmet need in identifying patients with CKD and managing risk factors for disease progression [4,5,6].
Glomerulonephritis (GN) is a leading cause of CKD, with CKD prevalence continuing to increase [7]. In the United Kingdom, in paediatric and adult populations, GN is the second most common primary renal diagnosis in those commencing kidney replacement therapy [8]. Glomerulonephritis presents treatment challenges due to the many intricate immunopathogenic processes that remain to be fully characterised. At present, a kidney biopsy is required to identify histopathological lesions and patterns that correlate with a specific GN diagnosis. With advances in our appreciation of the heterogeneity of GN, it is believed that confirmatory histology does not uncover the immunopathogenesis of the active inflammatory process at play. Further, there remains a pressing need to expand our use of immunomodulatory drugs and discover new drugs as, to date, there is an ongoing dependence on glucocorticoids as the mainstay of treatment, exposing patients to their long-term side effects [9,10,11].
To strengthen our clinical diagnosis, it is imperative that we are confident of the molecular architecture of a diseased state. The “omics” refers to a group of scientific disciplines aiming to generate large quantities of data by characterising different layers of the biochemical composition of a biological system. The most utilised of these sciences are genomics, transcriptomics, proteomics and metabolomics. These approaches consolidate our understanding of disease pathogenesis and phenotypes and are becoming integral to translational precision medicine, with biomarker discovery now frequently based on omic data [12,13,14]. Through omics analysis, samples can be comprehensively characterised, and these results can be interpreted alongside the clinical data [15, 16].
In the case of kidney disease, both metabolomics and proteomics techniques have become well established, allowing blood, urine and tissue samples to be analysed [17]. Untargeted proteomics (also known as bottom-up or shotgun proteomics) analyses the protein composition of a biological system [18]. In many instances, these proteins can be modified with other chemical classes which impact the structure, function and stability of proteins and include; phosphate groups or carbohydrates known as glycans, the latter of which are well established and contribute to kidney disease [19].
Metabolomics refers to the characterisation of “small molecules” typically < 1000 Da in size that encompass key metabolic components such as amino acids, steroids, bile acids and organic acids, which comprise enzymatic substrates, cofactors and products [20]. Metabolomics has been widely applied to GN [21], acute kidney injury (AKI) [22] and the development of kidney cancer [23]. Lipidomics is a sub-category of the metabolome, characterising all lipids within a biofluid or tissue, such as phospholipids, triacylglycerides, eicosanoids and fatty acids. Lipidome dysregulation, in particular, has been linked to CKD and cardiovascular risk [24, 25]. It is becoming increasingly acknowledged that the wealth of information to be discovered through multi-omic analysis, including proteomics and metabolomics, can lead to the development of precision medicine in CKD [26, 27].
The aim of this study was to perform a systematic literature review to summarise the current application of proteomic and metabolomic techniques for GN to identify strengths and areas of unmet need.
Methods
This systematic review was registered in PROSPERO (CRD42023442092). The inclusion criteria were patients of any age, sex or ethnicity who had a histological or genetic diagnosis of GN as per the Kidney Disease: Improving global outcomes (KDIGO) criteria [28]. The methods included were; the use of one of the three most frequently applied analytical techniques: liquid chromatography–mass spectrometry; gas chromatography–mass spectrometry or proton nuclear magnetic resonance untargeted metabolomic or proteomic analysis; and any human biofluid or tissue. Studies based on therapeutic drug monitoring were excluded.
The PICO framework for the systematic review was:
Population: Patients of any age, sex or ethnicity who had a histological or genetic diagnosis of GN as per the KDIGO criteria [28]
Intervention: Untargeted metabolomics (including lipidomics) or proteomic analysis.
Comparator: Currently adopted lab techniques in clinical practice.
Outcome: Discovery of clinically relevant results that can change current practice.
Three online databases were searched on the 14th March, 2023: Cochrane, Ovid and Scopus.
The study designs included were meta-analyses, randomised control trials, cohort studies, case–control studies, cross-sectional studies and case series (n > 5). The filters applied to the search tool were; an original publication date between 2013 and 2023 (allowing for an inclusion period of 10 years), accessible in full text through the University of Liverpool, an abstract available in English with sufficient data for extraction. Studies that identified exogenous metabolites (such as those associated with ingested food products or drugs) as biomarkers were excluded alongside secondary data and animal studies. The reference lists of relevant literature were hand-searched to identify any additional eligible studies.
The search terms applied to the databases were;
(Glomerulonephritis) 0R (IgA nephropathy) OR (membranous nephropathy) OR (fsgs) OR (focal segmental glomerulosclerosis) OR (Nephrotic syndrome) OR (minimal change disease) OR (Lupus nephritis) OR (Membranoproliferative glomerulonephritis) OR (mpgn) OR (ANCA-associated vasculitis) OR (Antineutrophil cytoplasmic antibody associated vasculitis) OR (microscopic polyangiitis) OR (Mpa) OR (eosinophilic granulomatosis with polyangiitis) OR (egpa) OR (wegener's granulomatosis) OR (anti-GBM antibody) OR (anti-glomerular basement membrane) OR (goodpastures) AND (Omic) OR (Proteomics) OR (Metabolomics) OR (Lipidomics) OR (Mass spectrometry) OR (GC–MS) OR (NMR) OR (LC–MS).
Selection process
Four reviewers completed title screening independently: AC, ED, LO, and AR. Abstract screening and full text screening was completed by two reviewers (AC and ED). At every level of review any conflicts were discussed and subsequently resolved. Duplicate results were screened electronically by Rayaan software, and any further remaining duplicates were manually removed after cross-checking. The Critical Appraisal Skills Programme (CASP), Cohort study checklist was applied to each included study to evaluate the quality of the study to determine the risk of bias [29].
Data collection and analysis
Descriptive analysis was applied to the data collected from the included studies and presented in tabular form. The data outcomes extracted from each study were; first named author, country of study, publication year, study design, subtype GN, cohort demographics, sample analysed, analytical technique utilised and key outcomes. Area under curve (AUC) or p-value was presented for those studies that identified potential biomarkers or statistically significant molecular discoveries.
Sex distribution was converted to a percentage of males. The average age was calculated from those studies which provided complete demographic data. Study demographics were split into the named GN subtype, disease control or healthy control where applicable. Incomplete data values were recorded as NA.
The study was split into two groups depending on the omic analysis utilised: metabolomics (including lipidomics) and proteomics.
Results
Data extraction
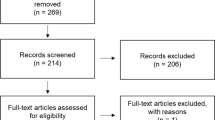
An online database search was completed in March 2023 and yielded 1081 papers. A total of 269 duplicates were identified and removed. The remaining 812 records were screened by abstract and a subsequent 109 were included for full-text review. The final number of papers included for review was 27. No further papers were included from screening reference lists. The process of article selection is shown in Fig. 1.
A flow diagram of the screening process. Literature search performed on four databases returned a total of 1081 papers. Following removal of duplicates, 812 papers were screened. After screening by an initial and a second independent researcher, a total of 27 studies were included in the systematic review
Quality assessment
The CASP checklist was applied to all included studies. The checklist highlighted the risk of bias in those studies without a control cohort and that prior exposure to immunosuppression was an important confounding factor that was not accounted for in some studies.
Metabolomics and lipidomics
A total of 9 included studies were based on metabolomics (n = 8) and lipidomics (n = 1) and used different analytical platforms: proton nuclear magnetic resonance (n = 6), gas chromatography–mass spectrometry n = (2), liquid chromatography–mass spectrometry (n = 2). The total population cohort included 1,196 patients (Average cohort size 133, range 13–497), of whom 287 (24%) were healthy controls. The overall sex distribution included 45% males with an average age of 38 years (range 6–50). The samples analysed were urine (n = 7), blood (n = 1) and both blood and urine samples (n = 1). IgA nephropathy (IgAN) and IgA Vasculitis (IgAV) were the most frequently investigated GN (n = 4), followed by focal segmental glomerulosclerosis (FSGS) (n = 3) membranous nephropathy (MN) (n = 2), lupus nephritis (LN) (n = 2), minimal change disease (MCD) (n = 2).
Two studies investigating IgAN, did not include a healthy cohort of patients for comparison. All studies were cross-sectional cohort studies.
A summary of the key results from the studies using metabolomics and lipidomics in GN is presented in Table 1.
Potential diagnostic biomarkers
Four papers identified potential diagnostic biomarkers for specific GN subtypes. The Taherkhani et al. [35] study included 79 MN patients, 83 disease controls and 53 healthy controls with an average age of 39 years. A panel of seven lipid metabolites in urine was identified that could differentiate idiopathic MN from healthy controls and disease controls with an AUC 1.0. This study used two different analytical techniques to analyse samples, gas chromatography–mass spectrometry and proton nuclear magnetic resonance. The proposed metabolites panel reflected those of significance across both analytical strategies. Park et al.[32], in a study of 201 IgAN compared with 160 disease controls and 136 healthy controls with an average age of 43 years, analysed urine samples using proton nuclear magnetic resonance and validated the results with liquid chromatography–mass spectrometry. A model was developed using identified biomarkers alongside demographics (age and sex), kidney parameters (estimated glomerular filtration rate (eGFR), urine protein: creatinine ratio), and mean arterial pressure. This model was diagnostic of IgAN with AUC 0.931.
Disease phenotype and risk of progression
Two studies without healthy control cohorts analysed samples from IgAN patients. Zhang et al. [30] identified two lipid-related molecules, Choline and Cis-vaccenic acid, present in both serum and urine, that could distinguish IgAV from IgAN. Forty-six IgAV and 44 IgAN patients were included, with an average age of 37 years. A panel of choline and cis-vaccenic acid gave an AUC of 0.927 in serum and 0.7243 in urine, which could distinguish between disease phenotypes.
Kalantari et al. [31] studied a cohort of 13 IgA patients with an average age of 33 years that were separated into mild and severe groups depending on Oxford biopsy classification. The aim was to establish any urinary biomarkers that could correlate with the histological classification of disease. Nine metabolites were positively correlated with proteinuria, and three were negatively correlated with proteinuria. The results also identified that phenylalanine metabolism was a significant metabolic pathway that was altered and correlated with disease progression.
Distinguishing between systemic lupus erythematosus (SLE) and LN and a healthy control using lipidomic analysis of serum samples was investigated by Guleria et al. [33], identifying an altered lipid metabolome that could identify LN activity. Guleria et al. [33] studied 22 SLE, 40 LN and 30 healthy controls with an average age of 30 years. Elevated serum levels of low-density/very low-density lipoproteins (triglyceride and fatty acid) and decreased serum levels of acetate were apparent in LN. Data analysis included investigating the correlation between the discriminatory serum metabolites and SLE disease activity index (SLEDAI) for the SLE group, but no significant correlation was observed.
Proteomics
A total of 18 studies were included, all studies utilised liquid chromatography–mass spectrometry. The cohort included 622 patients (average cohort size 35, range 10–103) as GN or disease controls and 135 healthy controls. The average sex distribution across all study cohorts was 55% male, with an average age of 32 years (Range 4–60). The samples analysed were urine (n = 11), biopsy (n = 6) and blood (n = 2); one study analysed both blood and urine samples. IgAN was the most frequently investigated GN (n = 7), followed by MN (n = 4), LN (n = 4), FSGS (n = 5), and MCD (n = 1).
A total of 15 studies were cross-sectional cohort studies alongside three longitudinal studies aimed to identify responses to treatment. Seven studies had a healthy control cohort. Three studies did not have any demographic data. Two studies exclusively investigated a paediatric population with a cohort size of 61 and 18, respectively.
A summary of the key results from the proteomic studies in GN is presented in Table 2.
Potential diagnostic biomarkers
The primary aim of 14 studies was to identify potential new biomarkers. Rood et al. [52] analysed urine samples in a small cohort of 5 MN patients and discovered LIMP-2 peptides (p < 0.01), a potential biomarker for Idiopathic MN compared to healthy controls and FSGS patients. Pang et al. [50] also compared a cohort of idiopathic MN, both Anti-phospholipase A2 receptor antibody- (PLA2R)negative (32) and positive (31), to healthy controls (32). Two potential biomarkers were highlighted, alpha-1-antitrypsin and afamin with follow up confirmation analysis performed using western blot analysis.
Samavat et al. [39] aimed to identify biomarkers for IgAN in urine samples of 13 patients alongside 8 healthy controls with an average age of 34 years. Ten proteins were either up-regulated or down-regulated compared to healthy controls, but no clear statistically significant biomarker was found. The outcome was similar for Xue et al. [40], again investigating IgAN using serum samples from 60 patients and 43 healthy controls, where 12 proteins were identified and validated but there were no statistically significant differences between groups to suggest a clear biomarker.
Disease phenotype and risk of progression
Turnier et al. [46] carried out a paediatric study of LN using urine samples of 61 patients obtained within a month of renal biopsy. A potential biomarker was unveiled; α1-antichymotrypsin, encoded by the SERPINA3 gene, was found to have a moderate positive association (p = 0.005) with the histological disease severity measure National Institutes of Health Activity Index (NIH-AI). Mao et al. [49] performed proteomics on LN biopsy samples of 10 patients with an average age of 33 years. A previously researched protein, NEU1 [57], showed increased expression in patients with a higher chronicity index of disease and multivariate Cox regression analysis (HR, 6.462 (95% CI 1.025–40.732), p = 0.047) for renal prognosis. NEU1 was also found to be present in greater abundance in the urine samples of those patients.
Three further studies analysed a cohort of patients with IgAN. Paunas et al. [44] retrospectively analysed the biopsy samples of two cohorts of patients; 10 with no disease progression defined as no end-stage kidney disease (ESKD) 10 years post biopsy and 9 with ESKD 10 years post biopsy. Periostin showed promise as a novel and important risk marker of disease progression with AUC 0.91. Furthermore, stronger periostin staining by immunohistochemistry was subsequently seen in the progressive IgAN patients. Kalantari et al. [42] analysed urine samples from IgAN patients of different severity based on biopsy findings. Although no biomarkers were identified from 232 proteins, the results provided insight on the possible pathogenic pathways linked with disease progression. A further paediatric study by Fang et al. [41] investigated urine of 19 IgAN and 19 IgAV patients with an average age of 9 years and compared them to healthy controls. The metabolic pathways associated with the proteins identified were the complement and coagulation cascades and platelet activation. A1BG and AFM proteins were significantly increased in children with IgAN and IgAV but could not distinguish the two disease phenotypes.
Treatment response
Three longitudinal studies applied proteomic analysis to establish a response to treatment. Ghasemi et al. [48] collected blood and urine samples from 19 LN patients at the time of renal biopsy. The patients were followed up for up to four years, the primary outcome being disease remission. Twenty plasma proteins and ten urine proteins could be identified as potential biomarkers.
Kalantari et al. [53] utilised urine samples from a cohort of 10 patients, six steroid-sensitive and four steroid-resistant, with FSGS at the time of biopsy. Steroid resistance was defined as failure to respond to the steroid regimen at eight weeks. Results showed a drastic fold change in two proteins, APOA-1 and MXRA8.
Ni et al. [55], carried out a paediatric study of 18 FSGS patients, 7 steroid-sensitive and 11 steroid-resistant, utilising biopsy samples with steroid resistance defined at six weeks. Two proteins, LAMP1 and ACSL4, previously described in the literature, were raised in steroid-resistant disease. These proteins were subsequently stained on biopsy samples to confirm their presence.
Discussion
This review outlines the current utility of metabolomics and proteomics in children and adults with a histological diagnosis of GN. We aimed to establish the existing evidence and identify areas of unmet need. We reviewed 27 studies in total: 9 using metabolomic and lipidomic analysis, 18 using proteomics. The most frequently studied GN disease was IgAN, reflecting its place as the most prevalent primary glomerular disease worldwide [58]. Urine was the most frequently investigated sample type, and the study cohorts had an average cohort size of 113 in metabolomics and 35 in proteomics. The average age of participants was 35 years, and only four studies included a paediatric cohort.
The primary aim of most studies was to identify new diagnostic biomarkers. The aim is to produce less invasive and more rapid diagnostics alongside personalised medicine. To date, this area has been dominated by genomic discoveries in cancer [59, 60]. The most notable development in GN has been made in MN whereby Beck et al. [61] discovered a novel antibody M-type phospholipase A (2) receptor (PLA2R) using mass spectrometry, which is now widely used in clinical practice. This review highlighted several small-scale studies in nephrology; however, large studies were sparse. Tofte et al. [62] conducted a large multi-centre study of 1775 participants with type 2 diabetes and no proteinuria to validate the use of CKD273, a urinary biomarker composed of 273 peptides previously identified through proteomic analysis of CKD cohorts [63]. A scoring system was created based on CKD273 results that equate to the risk of developing proteinuria. This longitudinal study showed that patients with a high-risk score from the urinary biomarker CKD273 correlated with the development of proteinuria over a median of 2.5 years, independent of clinical characteristics [62].
Confirming disease activity and prognosis was another aim identified in this review applied to LN- and IgA-related nephropathy cohorts. In IgA patients, the aim was to identify urinary biomarkers that reflect the histological classification of disease and establish biomarkers from kidney histology that can correlate with predicting disease progression. IgAN has been researched using omic methods, and we have better insight into the pathogenesis and immunomodulatory changes that are key in this disease pattern. However, studies thus far have not yet succeeded in identifying biomarkers that can be utilised to develop precision nephrology and achieve personalised therapy [64, 65]. A recent study by Pitcher et al.[66] of long term outcomes in IgAN based on a UK registry showed a median (95% CI) kidney survival of 10.8 (10.0 to 12.0) years. At present, the role of immunosuppression is unclear in different disease phenotypes, and further omic analysis may introduce a better understanding of its benefits at certain stages of disease activity. Alonso et al. [67] analysed urinary metabolites using nuclear magnetic resonance across a range of autoimmune diseases including SLE and Crohn's. The results showed a clear pattern of metabolites that correlated with disease activity as per the currently used disease activity scores [68]. Without the advent of specific disease activity biomarkers, we are delaying the early initiation or alteration of treatment regimens that can affect the patients' long-term outcomes.
The mainstay of treatment in GN requires immunosuppression to achieve and sustain clinical remission. Whilst there are globally accepted guidelines for managing these diseases [28] there is still an unmet need in delivering personalised treatment to patients. Immunosuppressive regimens in GN are often dependent on glucocorticoids, which are increasingly highlighted as having significant long-term effects, including increasing the risk of cardiovascular events [69, 70]. The adoption of multi-omic data has led to the capture of a vast amount of data in cancer with the ability to predict prognosis and treatment response [71, 72]. However, this data's most successful progress and clinical adoption has been from genomic analysis. In prostate cancer, the development of the Decipher score has led to the use of genomic data to accurately predict which cancer will behave more aggressively and therefore inform treatment choices [73, 74]. Results from cancer genomics analyses have led to the creation of the 'Cancer Genome Atlas', which documents the molecular features of an array of cancers. This invaluable data tool can aid the classification of cancers and could represent a platform for further research to develop targeted treatment for specific cancer sub-types [75].
It has long been established that cardiovascular disease is the leading cause of mortality in individuals diagnosed with CKD [76,77,78,79]. To date, we are continuing to unravel the interplay of disease processes and sociodemographic risk factors that accelerate atherosclerosis in CKD populations and the unique pro-inflammatory states associated [80, 81]. Lipidomic analysis has successfully shown that changes within the lipidome interplay and contribute to the pro-inflammatory state leading to atherosclerosis in cardiovascular disease and atherosclerosis in CKD [82, 83].
The main limitation of the included studies is the sample size, especially in proteomic analysis, where the average cohort size was only 35. To produce statistically significant results and uncover potential new biomarkers, these studies should aim to recruit larger cohorts. Multiple tools have been developed to support the multi-omic analysis power calculation for multi-omic analysis [84]. The technology needed for multi-omic analysis requires expensive infrastructure, which may not be so widely accessible, with finances limiting the sample size. The papers were heterogeneous in their methodology and data analysis, making direct comparison of their outcomes and significance more challenging. Moreover, only four studies included paediatric patients, perhaps representing cohorts with a more active phenotype and fewer co-morbidities.
Conclusion
This review details the current metabolomic and lipidomic analysis landscape in GN. There is clear evidence that the application of omic techniques through the analysis of blood, urine and kidney histology can elucidate the immunopathogenesis of GN and contribute to the development of precision medicine in nephrology.
References
Kovesdy CP (2022) Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 12(1):7–11
Bikbov B et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395(10225):709–733
Carney EF (2020) The impact of chronic kidney disease on global health. Nat Rev Nephrol 16(5):251
Nissenson AR et al (2001) Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol 12(8):1713–1720
Ma I et al (2018) Sociodemographic associations with abnormal estimated glomerular filtration rate (eGFR) in a large Canadian city: a cross-sectional observation study. BMC Nephrol 19(1):198
Stevens PE et al (2007) Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 72(1):92–99
Hu J et al (2023) Global, regional, and national burden of CKD due to glomerulonephritis from 1990 to 2019: a systematic analysis from the global burden of disease study 2019. Clin J Am Soc Nephrol 18(1):60–71
Registry, U.R. UK Renal Registry (2022) UK renal registry 24th annual report—data to 31/12/2020. 2022. Accessed 06 Aug 2023]. Available from: https://ukkidney.org/audit-research/annual-report.
Chadban SJ, Atkins RC (2005) Glomerulonephritis. Lancet 365(9473):1797–1806
Anders H-J, Jayne DRW, Rovin BH (2016) Hurdles to the introduction of new therapies for immune-mediated kidney diseases. Nat Rev Nephrol 12(4):205–216
Anders HJ et al (2023) Glomerulonephritis: immunopathogenesis and immunotherapy. Nat Rev Immunol 23(7):453–471
Hartl D et al (2021) Translational precision medicine: an industry perspective. J Transl Med 19(1):245
Subramanian I et al (2020) Multi-omics data integration, interpretation, and its application. Bioinform Biol Insights 14:1177932219899051
Schmidt DR et al (2021) Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin 71(4):333–358
Babu M, Snyder M (2023) Multi-omics profiling for health. Mol Cell Proteomics 22(6):100561
Yamada R et al (2021) Interpretation of omics data analyses. J Hum Genet 66(1):93–102
Kang M, Ko E, Mersha TB (2022) A roadmap for multi-omics data integration using deep learning. Brief Bioinform 23(1):bbab454
Verrills NM (2006) Clinical proteomics: present and future prospects. Clin Biochem Rev 27(2):99–116
Dotz V et al (2021) O- and N-glycosylation of serum immunoglobulin a is associated with iga nephropathy and glomerular function. J Am Soc Nephrol 32(10):2455–2465
Kell DB et al (2005) Metabolic footprinting and systems biology: the medium is the message. Nat Rev Microbiol 3(7):557–565
Zhao Y-Y (2013) Metabolomics in chronic kidney disease. Clin Chim Acta 422:59–69
Chetwynd AJ et al (2017) Nanoflow-nanospray mass spectrometry metabolomics reveals disruption of the urinary metabolite profiles of HIV-positive patients on combination antiretroviral therapy. JAIDS J Acquir Immune Defic Syndr 74(2):e45-53
Guida F et al (2021) The blood metabolome of incident kidney cancer: a case-control study nested within the MetKid consortium. PLoS Med 18(9):e1003786
Han X, Gross RW (2022) The foundations and development of lipidomics. J Lipid Res 63(2):100164
Baek J et al (2022) Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat Rev Nephrol 18(1):38–55
Eddy S, Mariani LH, Kretzler M (2020) Integrated multi-omics approaches to improve classification of chronic kidney disease. Nat Rev Nephrol 16(11):657–668
Provenzano M et al (2021) OMICS in chronic kidney disease: focus on prognosis and prediction. Int J Mol Sci 23(1):336
Rovin BH et al (2021) Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int 100(4):753–779
Programme, C.A.S. CASP Cohort study Checklist. 2002. Accessed 06 aug 2023. Available from: https://casp-uk.net/images/checklist/documents/CASP-Cohort-Study-Checklist/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf
Zhang Q et al (2021) Serum-urine matched metabolomics for predicting progression of henoch-schonlein purpura nephritis. Front Med. https://doi.org/10.3389/fmed.2021.657073
Kalantari S et al (2017) 1 H NMR-based metabolomics study for identifying urinary biomarkers and perturbed metabolic pathways associated with severity of IgA nephropathy: a pilot study. Magn Reson Chem 55(8):693–699
Park S et al (2021) Comprehensive metabolomic profiling in early IgA nephropathy patients reveals urine glycine as a prognostic biomarker. J Cell Mol Med 25(11):5177–5190
Guleria A et al (2016) NMR based serum metabolomics reveals a distinctive signature in patients with Lupus Nephritis. Sci Rep 6:35309
Kalantari S et al (2019) Metabolomics approach reveals urine biomarkers and pathways associated with the pathogenesis of lupus nephritis. Iran J Basic Med Sci 22(11):1288–1295
Taherkhani A et al (2019) Metabolomic analysis of membranous glomerulonephritis: identification of a diagnostic panel and pathogenic pathways. Arch Med Res 50(4):159–169
Hao X et al (2013) Distinct metabolic profile of primary focal segmental glomerulosclerosis revealed by NMR-based metabolomics. PLoS ONE 8(11):e78531
Erkan E et al (2016) Distinct urinary lipid profile in children with focal segmental glomerulosclerosis. Pediatr Nephrol 31(4):581–588
Lee JE et al (2016) Systematic biomarker discovery and coordinative validation for different primary nephrotic syndromes using gas chromatography-mass spectrometry. J Chromatogr A 1453:105–115
Samavat S et al (2015) Diagnostic urinary proteome profile for immunoglobulin a nephropathy. Iran J Kidney Dis 9(3):239–248
Xue D et al (2023) Serum proteomic analysis by nanoflow LC-MS/MS-based proteomics in iga chronic kidney disease. Clin Lab 69(3):01
Fang X et al (2021) Use of liquid chromatography-tandem mass spectrometry to perform urinary proteomic analysis of children with IgA nephropathy and Henoch-Schonlein purpura nephritis. J Proteomics 230:103979
Kalantari S et al (2013) Urinary prognostic biomarkers and classification of IgA nephropathy by high resolution mass spectrometry coupled with liquid chromatography. PLoS ONE [Electronic Resource] 8(12):e80830
Mucha K et al (2014) Complement components, proteolysis-related, and cell communication-related proteins detected in urine proteomics are associated with IgA nephropathy. Pol Arch Med Wewn 124(7):380–386
Paunas FTI et al (2022) Proteomic signature of tubulointerstitial tissue predicts prognosis in IgAN. BMC Nephrol 23(1):118
Kawata N et al (2020) Proteomics of human glomerulonephritis by laser microdissection and liquid chromatography-tandem mass spectrometry. Nephrology 25(4):351–359
Turnier JL et al (2019) Discovery of SERPINA3 as a candidate urinary biomarker of lupus nephritis activity. Rheumatology 58(2):321–330
Chen YY et al (2022) Proteomic profiling of kidney samples in patients with pure membranous and proliferative lupus nephritis. Lupus 31(7):837–847
Ghasemi M et al (2021) Predictive biomarker panel in proliferative lupus nephritis- two-dimensional shotgun proteomics. Iran J Kidney Dis 1(2):121–133
Mao Z et al (2021) Discovery of NEU1 as a candidatedone. renal biomarker for proliferative lupus nephritis chronicity. Lupus Sci Med 8(1):12
Pang L et al (2018) Urine proteomics of primary membranous nephropathy using nanoscale liquid chromatography tandem mass spectrometry analysis. Clin Proteom 15(1):1–15
Li S et al (2022) Label-free quantitative proteomic and phosphoproteomic analyses of renal biopsy tissues in membranous nephropathy. Proteomics Clin Appl 16(1):e2000069
Rood IM et al (2015) Increased expression of lysosome membrane protein 2 in glomeruli of patients with idiopathic membranous nephropathy. Proteomics 15(21):3722–3730
Kalantari S et al (2014) Predictive urinary biomarkers for steroid-resistant and steroid-sensitive focal segmental glomerulosclerosis using high resolution mass spectrometry and multivariate statistical analysis. BMC Nephrol 15:141
Kalantari S et al (2014) Urinary prognostic biomarkers in patients with focal segmental glomerulosclerosis. Nephro-Urology Mon 6(2):e16806
Ni J et al (2022) Comparative proteomic analysis of children FSGS FFPE tissues. BMC Pediatr 22(1):707
Chebotareva NV et al (2022) Potential urine proteomic biomarkers for focal segmental glomerulosclerosis and minimal change disease. Int J Mol Sci 23(20):20
Nowling TK et al (2015) Renal glycosphingolipid metabolism is dysfunctional in lupus nephritis. J Am Soc Nephrol 26(6):1402–1413
Schena FP, Nistor I (2018) Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol 38(5):435–442
Chen G et al (2023) Integrative analysis of multi-omics data for liquid biopsy. Br J Cancer 128(4):505–518
Alix-Panabières C, Pantel K (2021) Liquid biopsy: from discovery to clinical application. Cancer Discov 11(4):858–873
Beck LH Jr et al (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361(1):11–21
Tofte N et al (2020) Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol 8(4):301–312
Good DM et al (2010) Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics 9(11):2424–2437
Schena FP et al (2018) Omics studies for comprehensive understanding of immunoglobulin A nephropathy: state-of-the-art and future directions. Nephrol Dial Transplant 33(12):2101–2112
Mucha K, Pac M, Pączek L (2023) Omics are getting Us closer to understanding IgA nephropathy. Arch Immunol Ther Exp (Warsz) 71(1):12
Pitcher D et al (2023) Long-term outcomes in IgA nephropathy. Clin J Am Soc Nephrol 18(6):727–738
Alonso A et al (2016) Urine metabolome profiling of immune-mediated inflammatory diseases. BMC Med 14(1):133
Ceccarelli F et al (2015) Assessment of disease activity in systemic lupus erythematosus: lights and shadows. Autoimmun Rev 14(7):601–608
Huscher D et al (2009) Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis 68(7):1119–1124
Moya FB, Galindo LFP, de la Peña MG (2016) Impact of chronic glucocorticoid treatment on cardiovascular risk profile in patients with systemic lupus erythematosus. J Clin Rheumatol 22(1):8–12
Olivier M et al (2019) The need for multi-omics biomarker signatures in precision medicine. Int J Mol Sci 20(19):4781
Shmatko A et al (2022) Artificial intelligence in histopathology: enhancing cancer research and clinical oncology. Nature Cancer 3(9):1026–1038
Erho N et al (2013) Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE 8(6):e66855
Jairath NK et al (2021) A systematic review of the evidence for the decipher genomic classifier in prostate cancer. Eur Urol 79(3):374–383
Hutter C, Zenklusen JC (2018) The cancer genome atlas: creating lasting value beyond its data. Cell 173(2):283–285
Jankowski J et al (2021) Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 143(11):1157–1172
Matsushita K et al (2022) Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol 18(11):696–707
Matsushita K et al (2015) Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3(7):514–525
Sarnak MJ et al (2003) Kidney disease as a risk factor for development of cardiovascular disease. Circulation 108(17):2154–2169
Balla S, Nusair MB, Alpert MA (2013) Risk factors for atherosclerosis in patients with chronic kidney disease: recognition and management. Curr Opin Pharmacol 13(2):192–199
Valdivielso JM et al (2019) Atherosclerosis in chronic kidney disease: more, less, or just different? Arterioscler Thromb Vasc Biol 39(10):1938–1966
Ding M, Rexrode KM (2020) A review of lipidomics of cardiovascular disease highlights the importance of isolating lipoproteins. Metabolites 10(4):163
Tracz J, Luczak M (2021) Applying proteomics and integrative “Omics” strategies to decipher the chronic kidney disease-related atherosclerosis. Int J Mol Sci 22(14):7492
Tarazona S et al (2020) Harmonization of quality metrics and power calculation in multi-omic studies. Nat Commun 11(1):3092
Funding
This work was supported by the Wellcome Trust [219574/Z/19/Z]; and the Faculty of Health and Life Sciences, University of Liverpool.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This systematic review is based on previously published peer-reviewed work and does not require any further ethical approvals.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Davies, E., Chetwynd, A., McDowell, G. et al. The current use of proteomics and metabolomics in glomerulonephritis: a systematic literature review. J Nephrol (2024). https://doi.org/10.1007/s40620-024-01923-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40620-024-01923-w