Abstract
Introduction
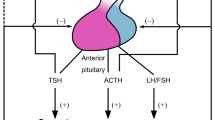
Pituitary function is influenced by several drugs, including anti-depressant, opioids, glucocorticoids, chemotherapeutic agents, immunomodulators and the newly developed tyrosine kinase inhibitors. In most instances, treatment with these drugs negatively affects pituitary function, but in rare cases an activation of specific hypothalamic-pituitary axes may be observed. Several of the observed pituitary side effects are reversible after drug withdrawal, but pituitary function deficiency may persist long-term. In addition to the well known drugs, recent evidence shows that also non-steroidal anti-inflammatory drugs impair gonadal axis at pituitary level, while antipsychotic phenothiazines alter TSH response to TRH and TSH levels. Atypical antipsychotics may decrease TRH-stimulated TSH. Tricyclic antidepressant drugs interfere with the hypothalamo-pituitary-thyroid axis by decreasing TSH response to TRH. Anabolic–androgenic steroids, marijuana, cocaine, methamphetamines, and opioid narcotics negatively impact fertility, also acting at hypothalamic-pituitary level.
Conclusions
Many of the drugs administered routinely in the intensive care unit significantly impact the hypothalamic-pituitary axis. Therefore, an increased awareness on pituitary side effects of drugs commonly used in clinical practice is necessary in order to rule out possible pharmacological interference when assessing patients with pituitary deficiencies.
Similar content being viewed by others
References
Tollin SR (2000) Use of the dopamine agonists bromocriptine and cabergoline in the management of risperidone-induced hyperprolactinemia in patients with psychotic disorders. J Endocrinol Invest 23:765–770
Berardelli R, Margarito E, Ghiggia F, Picu A, Balbo M, Bonelli L, Giordano R, Karamouzis I, Bo M, Ghigo E, Arvat E (2010) Neuroendocrine effects of citalopram, a selective serotonin re-uptake inhibitor, during lifespan in humans. J Endocrinol Invest 33:657–662. doi:10.3275/6994
Bou Khalil R, Richa S (2011) Thyroid adverse effects of psychotropic drugs: a review. Clin Neuropharmacol 34:248–255. doi:10.1097/WNF.0b013e31823429a7
Thomas Z, Bandali F, McCowen K, Malhotra A (2010) Drug-induced endocrine disorders in the intensive care unit. Crit Care Med 38:S219–S230. doi:10.1097/CCM.0b013e3181dda0f2
Nicoloff JT, Fisher DA, Appleman MD Jr (1970) The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 49:1922–1929
Ji K, Liu X, Lee S, Kang S, Kho Y, Giesy JP, Choi K (2013) Effects of non-steroidal anti-inflammatory drugs on hormones and genes of the hypothalamic-pituitary-gonad axis, and reproduction of zebrafish. J Hazard Mater 15(254–255):242–251. doi:10.1016/j.jhazmat.2013.03.036
Zawatski W, Lee MM (2013) Male pubertal development: are endocrine-disrupting compounds shifting the norms? J Endocrinol 218:R1–R12. doi:10.1530/JOE-12-0449
Mantovani A (2006) Risk assessment of endocrine disrupters. The role of toxicological studies. Ann N Y Acad Sci 1076:239–252
Faber KA, Hughes CL Jr (1993) Dose-response characteristics of neonatal exposure to genistein on pituitary responsiveness to gonadotropin releasing hormone and volume of the sexually dimorphic nucleus of the preoptic area (SDN-POA) in postpubertal castrated female rats. Reprod Toxicol 7:35–39
Takeda T, Fujii M, Hattori Y, Yamamoto M, Shimazoe T, Ishii Y, Himeno M, Yamada H (2014) Maternal exposure to dioxin imprints sexual immaturity of the pups through fixing the status of the reduced expression of hypothalamic gonadotropin-releasing hormone. Mol Pharmacol 85:74–82. doi:10.1124/mol.113.088575
Nogawa K, Kobayashi E, Okubo Y, Suwazono Y (2004) Environmental cadmium exposure, adverse effects, and preventative measures in Japan. Biometals 17:581–587
Lafuente A (2013) The hypothalamic-pituitary-gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches. Food Chem Toxicol 59:395–404. doi:10.1016/j.fct.2013.06.024
Fronczak CM, Kim ED, Barqawi AB (2012) The insults of illicit drug use on male fertility. J Androl 33:515–528. doi:10.2164/jandrol.110.011874
Vescovi PP, Pedrazzoni M, Michelini M, Maninetti L, Bernardelli F, Passeri M (1992) Chronic effects of marihuana smoking on luteinizing hormone, follicle-stimulating hormone and prolactin levels in human males. Drug Alcohol Depend 30:59–63
Bonetti A, Tirelli F, Catapano A, Dazzi D, Dei Cas A, Solito F, Ceda G, Reverberi C, Monica C, Pipitone S, Elia G, Spattini M, Magnati G (2008) Side effects of anabolic androgenic steroids abuse. Int J Sports Med 29:679–687
Abs R, Verhelst J, Maeyaert J, Van Buyten JP, Opsomer F, Adriaensen H, Verlooy J, Van Havenbergh T, Smet M, Van Acker K (2000) Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab 85:2215–2222
Brennan MJ (2013) The effect of opioid therapy on endocrine function. Am J Med 126:S12–S18
Tenhola H, Sinclair D, Alho H, Lahti T (2012) Effect of opioid antagonists on sex hormone secretion. J Endocrinol Invest 35:227–230. doi:10.3275/8181
Benavides M, Laorden ML, García-Borrón JC, Milanés MV (2003) Regulation of tyrosine hydroxylase levels and activity and Fos expression during opioid withdrawal in the hypothalamic PVN and medulla oblongata catecholaminergic cell groups innervating the PVN. Eur J Neurosci 17:103–112
Laorden ML, Castells MT, Martinez MD, Martinez PJ, Milanes MV (2000) Activation of c-fos expression in hypothalamic nuclei by mu- and kappa-receptor agonists. Correlation with catecholaminergic activity in the hypothalamic paraventricular nucleus. Endocrinology 141:1366–1376
Laorden ML, Fuertes G, Gonzalez-Cuello A, Milanes MV (2000) Changes in catecholaminergic pathways innervating paraventricular nucleus and pituitary-adrenal axis response during morphine dependence: implication of a1- and a2-adrenoceptors. J Pharmacol Exp Ther 293:578–584
Koob GF (1999) Corticotropin-releasing factor, norepinephrine and stress. Biol Psychiat 46:1167–1180
Heuser IJE, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Dettling M, Yassouridis A, Holsboer F (1996) Pituitary–adrenal system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. Am J Psychiat 153:93–99
Inder WJ, Prickett TCR, Mulder RT, Donald RA, Joyce PR (2001) Reduction in basal afternoon plasma ACTH during early treatment of depression with fluoxetine. Psychopharmacology 156:73–78
Rota E, Broda R, Cangemi L, Migliaretti G, Paccotti P, Rosso C, Torre E, Zeppegno P, Portaleone P (2005) Neuroendocrine (HPA axis) and clinical correlates during fluvoxamine and amitriptyline treatment. Psychiatry Res 133:281–284
Holsboer F (1995) Neuroendocrinology of mood disorders. In: Bloom FE, Kupfer DJ (eds) Phychopharmacology: the fourth generation of progress. Raven, New York, pp 957–969
Young EA, Altemus M, Lopez JF, Kocsis JH, Schatzberg AF, DeBattista C, Zubieta JK (2004) HPA axis activation in major depression and response to fluoxetine: a pilot study. Psychoneuroendocrinol 29:1198–1204
Heydendael W, Jacobson L (2010) Widespread hypothalamic–pituitary–adrenocortical axis-relevant and mood-relevant effects of chronic fluoxetine treatment on glucocorticoid receptor gene expression in mice. Eur J Neurosci 31:892–902
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C (2011) Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 16:1177–1188
Sampson SM (2001) Treating depression with selective serotonin reuptake inhibitors: a practical approach. Mayo Clin Proc 76:739–744
Barclay TS, Lee AJ (2002) Citalopram-associated SIADH. Ann Pharmacother 36:1558–1563
Goldberg JF (2000) New drugs in psychiatry. Emerg Med Clin North Am 18:211–231
Lakic N, Pericic D, Maney H (1986) Mechanism by which picrotoxin and a high dose of diazepam elevate plasma corticosterone level. Neuroendocrinology 43:331–335
Pohorecky LA, Cotler S, Carbone JJ, Robert P (1988) Factor modifying the effect of diazepam on plasma corticosterone levels in rats. Life Sci 43:2159–2167
De Boer SF, Der Gugten JV, Slangen JL (1991) Effects of chlordiazepoxide, umazenil and DMCM on plasma catecholamine and corticosterone concentrations in rats. Pharmacol Biochem Behav 38:13–19
Pivac N, Pericic D (1993) Inhibitory effect of diazepam on the activity of the hypothalamic-pituitary-adrenal axis in female rats. J Neural Transm 92:173–186
Vargas ML, Abella C, Hernandez J (2001) Diazepam increases the hypothalamic-pituitary-adrenocortical (HPA) axis activity by a cyclic AMP-dependent mechanism. Br J Pharmacol 133:1355–1361
De Boer SF, Van der Gugten J, Slangen JL (1990) Brain benzodiazepine receptor-mediated effects on plasma catecholamine and corticosterone concentrations in rats. Brain Res Bull 24:843–847
Schuckit MA, Hauger R, Klein JL (1992) Adrenocorticotropin hormone response to diazepam in healthy young men. Biol Psychiatry 31:661–669
Korbonits M, Trainer PJ, Edwards R, Besser GM, Grossman AB (1995) Benzodiazepines attenuate the pituitary-adrenal responses to corticotrophin-releasing hormone in healthy volunteers, but not in patients with Cushing’s syndrome. Clin Endocrinol Oxf 43:29–35
Giordano R, Berardelli R, Karamouzis I, D’Angelo V, Picu A, Zichi C, Fussotto B, Manzo M, Mengozzi G, Ghigo E, Arvat E (2013) Acute administration of alprazolam, a benzodiazepine activating GABA receptors, inhibits cortisol secretion in patients with subclinical but not overt Cushing’s syndrome. Pituitary 16:363–369
Mikkelsen JD, Søderman A, Kiss A, Mirza N (2005) Effects of benzodiazepines receptor agonists on the hypothalamic-pituitary-adrenocortical axis. Eur J Pharmacol 519:223–230
Yeung SC, Chiu AC, Vassilopoulou-Sellin R, Gagel RF (1998) The endocrine effects of nonhormonal antineoplastic therapy. Endocr Rev 19:144–172
Lodish MB (2013) Clinical review: kinase inhibitors: adverse effects related to the endocrine system. J Clin Endocrinol Metab 98:1333–1342. doi:10.1210/jc.2012-4085
Silberstein L, Johnston C, Bhagat A, Tibi L, Harrison J (2008) Pituitary apoplexy during induction chemotherapy for acute myeloid leukaemia. Br J Haematol 143:151. doi:10.1111/j.1365-2141.2008.07286.x
Błaut K, Wiśniewski P, Syrenicz A, Sworczak K (2006) Apoplexy of clinically silent pituitary adenoma during prostate cancer treatment with LHRH analog. Neuro Endocrinol Lett 27:569–572
Vitale G, Caraglia M, van Koetsveld PM, Maroni P, Marra M, Colao A, Lamberts SW, Cavagnini F, Hofland LJ (2009) Potential role of type I interferons in the treatment of pituitary adenomas. Rev Endocr Metab Disord 10:125–133. doi:10.1007/s11154-008-9083-3
Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH (2003) Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry 160:1342–1345
Andreassen M, Frystyk J, Miller KK, Kristensen LØ (2010) Interferon-β treatment associated with a biochemical profile suggestive of acromegaly. A case report of a patient treated for multiple sclerosis. Scand J Clin Lab Invest 70:519–522. doi:10.3109/00365513.2010.521256
Golden WM, Weber KB, Hernandez TL, Sherman SI, Woodmansee WW, Haugen BR (2007) Single-dose rexinoid rapidly and specifically suppresses serum thyrotropin in normal subjects. J Clin Endocrinol Metab 92:124–130
Terzolo M, Daffara F, Ardito A, Zaggia B, Basile V, Ferrari L, Berruti A (2014) Management of adrenal cancer: a 2013 update. J Endocrinol Invest 37:207–217
Baudry C, Coste J, Bou Khalil R, Silvera S, Guignat L, Guibourdenche J, Abbas H, Legmann P, Bertagna X, Bertherat J (2012) Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol 167:473–481. doi:10.1530/EJE-12-0358
Gentilin E, Tagliati F, Terzolo M, Zoli M, Lapparelli M, Minoia M, Ambrosio MR, degli Uberti EC, Zatelli MC (2013) Mitotane reduces human and mouse ACTH-secreting pituitary cell viability and function. J Endocrinol 218:275–285. doi:10.1530/JOE-13-0210
Daffara F, De Francia S, Reimondo G, Zaggia B, Aroasio E, Porpiglia F, Volante M, Termine A, Di Carlo F, Dogliotti L, Angeli A, Berruti A, Terzolo M (2008) Prospective evaluation of mitotane toxicity in adrenocortical cancer patients treated adjuvantly. Endocr Relat Cancer 15:1043–1053. doi:10.1677/ERC-08-0103
Zatelli MC, Gentilin E, Daffara F, Tagliati F, Reimondo G, Carandina G, Ambrosio MR, Terzolo M, degli Uberti EC (2010) Therapeutic concentrations of mitotane (o, p’-DDD) inhibit thyrotroph cell viability and TSH expression and secretion in a mouse cell line model. Endocrinology 151:2453–2461. doi:10.1210/en.2009-1404
Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A (2007) Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 356:2372–2380
Gentilin E, Molè D, Gagliano T, Minoia M, Ambrosio MR, degli Uberti EC, Zatelli MC (2014) Inhibitory effects of mitotane on viability and secretory activity in mouse gonadotroph cell lines. Reprod Toxicol 45C:71–76. doi:10.1016/j.reprotox.2014.01.008
Faje AT, Nachtigall L, Wexler D, Miller KK, Klibanski A, Makimura H (2013) Central diabetes insipidus: a previously unreported side effect of temozolomide. J Clin Endocrinol Metab 98:3926–3931. doi:10.1210/jc.2013-2435
Braun D, Kim TD, le Coutre P, Köhrle J, Hershman JM, Schweizer U (2012) Tyrosine kinase inhibitors noncompetitively inhibit MCT8-mediated iodothyronine transport. J Clin Endocrinol Metab 97:E100–E105. doi:10.1210/jc.2011-1837
Ohba K, Takayama T, Matsunaga H, Matsushita A, Sasaki S, Oki Y, Ozono S, Nakamura H (2013) Inappropriate elevation of serum thyrotropin levels in patients treated with axitinib. Thyroid 23:443–448. doi:10.1089/thy.2012.0378
Haap M, Gallwitz B, Thamer C, Müssig K, Häring HU, Kanz L, Hartmann JT (2007) Symptomatic hypoglycemia during imatinib mesylate in a non-diabetic female patient with gastrointestinal stromal tumor. J Endocrinol Invest 30:688–692
Bansal D, Shava U, Varma N, Trehan A, Marwaha RK (2012) Imatinib has adverse effect on growth in children with chronic myeloid leukemia. Pediatr Blood Cancer 59:481–484. doi:10.1002/pbc.23389
Kebapcilar L, Bilgir O, Alacacioglu I, Payzin B, Bilgir F, Oner P, Sari I, Calan M, Binicier O (2009) Does imatinib mesylate therapy cause growth hormone deficiency? Med Princ Pract 18:360–363. doi:10.1159/000226288
Bilgir O, Kebapcilar L, Bilgir F, Sarì I, Oner P, Karaca B, Alacacioglu I (2010) Is there any relationship between imatinib mesylate medication and hypothalamic-pituitary-adrenal axis dysfunction? Int J Clin Pract 64:45–50. doi:10.1111/j.1742-1241.2008.01856.x
Kunz JS, Bannerji R (2005) Alemtuzumab-induced syndrome of inappropriate anti-diuretic hormone. Leuk Lymphoma 46:635–637
Liapis K, Apostolidis J, Charitaki E, Panitsas F, Harhalakis N, Nikiforakis E (2008) Syndrome of inappropriate secretion of antidiuretic hormone associated with imatinib. Ann Pharmacother 42:1882–1886. doi:10.1345/aph.1L410
Spada A (1998) Growth factors and human pituitary adenomas. Eur J Endocrinol 138:255–257
Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, Melmed S (2011) EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest 121:4712–4721. doi:10.1172/JCI60417
Dahlhoff M, Blutke A, Wanke R, Wolf E, Schneider MR (2011) In vivo evidence for epidermal growth factor receptor (EGFR)-mediated release of prolactin from the pituitary gland. J Biol Chem 286:39297–39306. doi:10.1074/jbc.M111.243493
Gagliano T, Filieri C, Minoia M, Buratto M, Tagliati F, Ambrosio MR, Lapparelli M, Zoli M, Frank G, degli Uberti EC, Zatelli MC (2013) Cabergoline reduces cell viability in non functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Pituitary 16:91–100. doi:10.1007/s11102-012-0380-1
Ortiz LD, Syro LV, Scheithauer BW, Ersen A, Uribe H, Fadul CE, Rotondo F, Horvath E, Kovacs K (2012) Anti-VEGF therapy in pituitary carcinoma. Pituitary 15:445–449. doi:10.1007/s11102-011-0346-8
Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F (2013) Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 98:1361–1375. doi:10.1210/jc.2012-4075
Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517–2526. doi:10.1056/NEJMoa1104621
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. doi:10.1056/NEJMoa1003466
Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, Royal RE, Topalian SL, Haworth LR, Levy C, Rosenberg SA, Sherry RM (2005) Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother 28:593–598
Min L, Vaidya A, Becker C (2012) Association of ipilimumab therapy for advanced melanoma with secondary adrenal insufficiency: a case series. Endocr Pract 18:351–355. doi:10.4158/EP11273.OR
Dillard T, Yedinak CG, Alumkal J, Fleseriu M (2010) Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 13:29–38. doi:10.1007/s11102-009-0193-z
Barnard ZR, Walcott BP, Kahle KT, Nahed BV, Coumans JV (2012) Hyponatremia associated with Ipilimumab-induced hypophysitis. Med Oncol 29:374–377. doi:10.1007/s12032-010-9794-7
Acknowledgments
This work was supported by grants from the Italian Ministry of Education, Research and University (FIRB RBAP11884 M, FIRB RBAP1153LS, 2010TYCL9B_002), Fondazione Cassa di Risparmio di Ferrara, in collaboration with Laboratorio in rete del Tecnopolo “Tecnologie delle terapie avanzate” (LTTA) of the University of Ferrara.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zatelli, M.C., Ambrosio, M.R., Bondanelli, M. et al. Pituitary side effects of old and new drugs. J Endocrinol Invest 37, 917–923 (2014). https://doi.org/10.1007/s40618-014-0133-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-014-0133-2