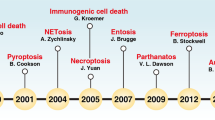
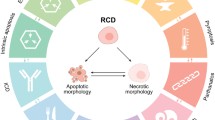
Abstract
One of the vital aspects of a cell is cell death to continue their normal cell turnover, propagation, proper development, and the maintenance of the immune system. Cell death is an essential process in the body as it promotes the removal of unwanted cells. It is the programmed culling of cells in entire eukaryotic development processes to survive and progress for the next generation. Molecular aberration in the process of apoptosis may have pathological manifestations, including cancer, neurodegenerative disorders, autoimmune disease, and ischemic damage. Classically, cell death is categorized primarily into four different types: apoptosis, autophagy, necrosis, and entosis; depending on cellular and molecular signatures governing the pathway involved. The purpose of this review is to compare and contrast the recent literature on cell death and to familiarize with the current state of knowledge on this topic. In summary, the hallmarks of various modes of cell death are thoroughly explained along with the other types of cell death such as ferroptosis, pyroptosis, necroptosis, and lysosomal-dependent cell death.








Similar content being viewed by others
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- DAMPs:
-
Damage-associated molecular patterns
- TNF-α:
-
Tumor necrosis factor-α
- NK:
-
Natural killer cells
- PCD:
-
Programmed cell death
- IAP:
-
Inhibitors of apoptosis proteins
- BIR:
-
Baculovirus IAP repeat
- RING:
-
C-terminal Ring zinc-finger domain
- CARD:
-
Caspase recruitment domain
- UBC:
-
C-terminal ubiquitin-conjugating domain
- LIMP:
-
Lysosomal integral membrane protein
- LAMP:
-
Lysosomal associated membrane protein
- LMP:
-
Lysosomal membrane permeabilization
- Hsp70:
-
Heat shock protein 70
- ROS:
-
Reactive oxygen species
- ADCD:
-
Autophagy-dependent cell death
- RIPK:
-
Receptor-interacting protein kinase
- MLKL:
-
Mixed lineage kinase domain-like protein
- CrmA:
-
Cytokine response modifier
- LPS:
-
Lipopolysaccharide
- FADD:
-
Fas-associated death domain
- BID:
-
BH3-interacting domain death agonist
- MON-P53:
-
Metal organic network-P53
- ECM:
-
Extracellular matrix
- FAK:
-
Focal adhesion kinase
- Mcl-1:
-
Myeloid cell leukemia sequence 1
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development homeostasis and disease. Cell. 2002;109(2):S97–107. https://doi.org/10.1016/s0092-8674(02)00704-3.
Sedger LM, Katewa A, Pettersen AK, et al. Extreme lymphoproliferative disease and fatal autoimmune thrombocytopenia in FasL and TRAIL double-deficient mice. Blood. 2010;115(16):3258–68. https://doi.org/10.1182/blood-2009-11-255497.
Lamhamedi-Cherradi S-E, Zheng S-J, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL-/- mice. Nat Immunol. 2003;4(3):255–60. https://doi.org/10.1038/ni894.
Su JH, Deng G, Cotman CW. Bax protein expression is increased in Alzheimer’s brain: correlations with DNA damage Bcl-2 expression and brain pathology. J Neuropathol Exp Neurol. 1997;56(1):86–93. https://doi.org/10.1097/00005072-199701000-00009.
Lu T, Aron L, Zullo J, et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature. 2014;507(7493):448–54. https://doi.org/10.1038/nature13163.
Nirmala JG, Lopus M. Cell death mechanisms in eukaryotes. Cell Biol Toxicol. 2020;36(2):145–64. https://doi.org/10.1007/s10565-019-09496-2.
Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26(4):605–16. https://doi.org/10.1038/s41418-018-0252-y.
• Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. https://doi.org/10.1038/s41418-017-0012-4. This review provided an updated classification of cell death subroutines focusing on mechanistic and essential aspects of the process.
Saïd-Sadier N, Ojcius DM. Alarmins inflammasomes and immunity. Biomed J. 2012;35(6):437–49. https://doi.org/10.4103/2319-4170.104408.
Zhou M, Aziz M, Wang P. Damage-associated molecular patterns as double-edged swords in sepsis. Antioxid Redox Signal. 2021;35(15):1308–23. https://doi.org/10.1089/ars.2021.0008 Published online March 30, 2021.
Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7(12):a006080. https://doi.org/10.1101/cshperspect.a006080.
Carella F, Feist SW, Bignell JP, De Vico G. Comparative pathology in bivalves: aetiological agents and disease processes. J Invertebr Pathol. 2015;131:107–20. https://doi.org/10.1016/j.jip.2015.07.012.
Yan G, Elbadawi M, Efferth T. Multiple cell death modalities and their key features (Review). World Acad Sci J. 2020;2(2):39–48. https://doi.org/10.3892/wasj.2020.40 Published online March 11, 2020.
Shalini S, Dorstyn L, Dawar S, Kumar S. Old new and emerging functions of caspases. Cell Death Differ. 2015;22(4):526–39. https://doi.org/10.1038/cdd.2014.216.
Chen Y, Hua Y, Li X, Arslan IM, Zhang W, Meng G. Distinct types of cell death and the implication in diabetic cardiomyopathy. Front Pharmacol. 2020;11:42. https://doi.org/10.3389/fphar.2020.00042.
Schweichel JU, Merker HJ. The morphology of various types of cell death in prenatal tissues. Teratology. 1973;7(3):253–66. https://doi.org/10.1002/tera.1420070306.
White C, Li C, Yang J, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7(10):1021–8. https://doi.org/10.1038/ncb1302.
Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wideranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–57. https://doi.org/10.1038/bjc.1972.33.
Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15(2):81–94. https://doi.org/10.1038/nrm3735.
Fulda S, Debatin K-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–811. https://doi.org/10.1038/sj.onc.1209608.
Ke FFS, Vanyai HK, Cowan AD, et al. Embryogenesis and adult life in the absence of intrinsic apoptosis effectors BAX BAK and BOK. Cell. 2018;173(5):1217–30. https://doi.org/10.1016/j.cell.2018.04.036.
Knudson CM, Tung KSK, Tourtellotte WG, Brown GAJ, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270(5233):96–9. https://doi.org/10.1126/science.270.5233.96.
Little GH, Flores A. Inhibition of programmed cell death by catalase and phenylalanine methyl ester. Comp Biochem Physiol Pt A Physiol. 1993;105(1):79–83. https://doi.org/10.1016/0300-9629(93)90176-5.
Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Mosby: Elsevier; 2008.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. https://doi.org/10.1080/01926230701320337.
Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16(6):2129–44. https://doi.org/10.7314/APJCP.2015.16.6.2129.
Kesavardhana S, Malireddi RKS, Kanneganti T-D. Caspases in cell death inflammation and pyroptosis. Annu Rev Immunol. 2020;38(1):567–95. https://doi.org/10.1146/annurev-immunol-073119-095439.
Poon IKH, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–80. https://doi.org/10.1038/nri3607.
Los M, de Craen MV, Penning LC, et al. Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature. 1995;375(6526):81–3. https://doi.org/10.1038/375081a0.
Sabbatini P, Han J, Chiou SK, Nicholson DW, White E. Interleukin 1 beta converting enzyme-like proteases are essential for p53-mediated transcriptionally dependent apoptosis. Cell Growth Differ. 1997;8(6):643–53.
Rathore S, Datta G, Kaur I, Malhotra P, Mohmmed A. Disruption of cellular homeostasis induces organelle stress and triggers apoptosis like cell-death pathways in malaria parasite. Cell Death Dis. 2015;6(7):e1803. https://doi.org/10.1038/cddis.2015.142.
Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5(11):897–907. https://doi.org/10.1038/nrm1496.
Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15(2):251–62. https://doi.org/10.1038/sj.cdd.4402244.
Osińska I, Popko K, Demkow U. Perforin: an important player in immune response. Cent Eur J Immunol. 2014;1:109–15. https://doi.org/10.5114/ceji.2014.42135.
Brentnall M, Rodriguez-Menocal L, De Guevara R, Cepero E, Boise LH. Caspase-9 caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14(1):32. https://doi.org/10.1186/1471-2121-14-32.
Morgan CW, Julien O, Unger EK, Shah NM, Wells JA. Turning ON Caspases with Genetics and Small Molecules. In: Ashkenazi A, Yuan J, Wells JA, editors. Methods in Enzymology, vol. 544. Amsterdam: Elsevier; 2014. p. 179–213. https://doi.org/10.1016/B978-0-12-417158-9.00008-X.
Ke B, Tian M, Li J, Liu B, He G. Targeting programmed cell death using small-molecule compounds to improve potential cancer therapy: anticancer compounds targeting cell death. Med Res Rev. 2016;36(6):983–1035. https://doi.org/10.1002/med.21398.
Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8(4):603–19. https://doi.org/10.18632/aging.100934.
Derakhshan A, Chen Z, Van Waes C. Therapeutic small molecules target inhibitor of apoptosis proteins in cancers with deregulation of extrinsic and intrinsic cell death pathways. Clin Cancer Res. 2017;23(6):1379–87. https://doi.org/10.1158/1078-0432.CCR-16-2172.
Silke J, Meier P. Inhibitor of Apoptosis (IAP) Proteins-modulators of cell death and inflammation. Cold Spring Harb Perspect Biol. 2013;5(2):a008730. https://doi.org/10.1101/cshperspect.a008730.
Liang J, Zhao W, Tong P, et al. Comprehensive molecular characterization of inhibitors of apoptosis proteins (IAPs) for therapeutic targeting in cancer. BMC Med Genomics. 2020;13(1):7. https://doi.org/10.1186/s12920-020-0661-x.
Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001;2(7):REVIEWS3009. https://doi.org/10.1186/gb-2001-2-7-reviews3009.
Land WG. Cell-autonomous (cell-intrinsic) stress responses. In: Damage-associated molecular patterns in human diseases. Cham: Springer International Publishing; 2018. p. 377–426. https://doi.org/10.1007/978-3-319-78655-1_18.
Galluzzi L, Baehrecke EH, Ballabio A, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36(13):1811–36. https://doi.org/10.15252/embj.201796697.
• Yim WW-Y, Mizushima N. Lysosome biology in autophagy Cell Discov. 2020;6(1):6. https://doi.org/10.1038/s41421-020-0141-7. The article provides a summary of current understanding on the behaviour of lysosomes during autophagy.
Wang F, Salvati A, Boya P. Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles. Open Biol. 2018;8(4):170271. https://doi.org/10.1098/rsob.170271.
Li W, Li J, Bao J. Microautophagy: lesser-known self-eating. Cell Mol Life Sci. 2012;69(7):1125–36. https://doi.org/10.1007/s00018-011-0865-5.
Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22(8):407–17. https://doi.org/10.1016/j.tcb.2012.05.006.
Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol. 2018;20(5):521–7. https://doi.org/10.1038/s41556-018-0092-5.
Klionsky DJ, Schulman BA. Dynamic regulation of macroautophagy by distinctive ubiquitin-like proteins. Nat Struct Mol Biol. 2014;21(4):336–45. https://doi.org/10.1038/nsmb.2787.
Condello M, Pellegrini E, Caraglia M, Meschini S. Targeting autophagy to overcome human diseases. IJMS. 2019;20(3):725. https://doi.org/10.3390/ijms20030725.
Shen H-M, Codogno P. Autophagic cell death: Loch Ness monster or endangered species? Autophagy. 2011;7(5):457–65. https://doi.org/10.4161/auto.7.5.14226.
Bialik S, Dasari SK, Kimchi A. Autophagy-dependent cell death – where how and why a cell eats itself to death. J Cell Sci. 2018;131(18):jcs215152. https://doi.org/10.1242/jcs.215152.
Cornillon S, Foa C, Davoust J, Buonavista N, Gross JD, Golstein P. Programmed cell death in Dictyostelium. J Cell Sci. 1994;107(Pt 10):2691–704.
Giusti C, Tresse E, Luciani M-F, Golstein P. Autophagic cell death: Analysis in Dictyostelium. Biochim Biophys Acta Mol Cell Res. 2009;1793(9):1422–31. https://doi.org/10.1016/j.bbamcr.2008.12.005.
Denton D, Aung-Htut MT, Kumar S. Developmentally programmed cell death in Drosophila. Biochim Biophys Acta Mol Cell Res. 2013;1833(12):3499–506. https://doi.org/10.1016/j.bbamcr.2013.06.014.
Ginet V, Spiehlmann A, Rummel C, et al. Involvement of autophagy in hypoxic-excitotoxic neuronal death. Autophagy. 2014;10(5):846–60. https://doi.org/10.4161/auto.28264.
Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and beclin 1 in mediating autophagy. Circ Res. 2007;100(6):914–22. https://doi.org/10.1161/01.RES.0000261924.76669.36.
Chen H-Y, White E. Role of autophagy in cancer prevention. Cancer Prev Res. 2011;4(7):973–83. https://doi.org/10.1158/1940-6207.CAPR-10-0387.
Ha S, Jeong S-H, Yi K, et al. Phosphorylation of p62 by AMP-activated protein kinase mediates autophagic cell death in adult hippocampal neural stem cells. J Biol Chem. 2017;292(33):13795–808. https://doi.org/10.1074/jbc.M117.780874.
Ouyang L, Zhang L, Liu J, et al. Discovery of a small-molecule bromodomain-containing protein 4 (BRD4) inhibitor that induces AMP-activated protein kinase-modulated autophagy-associated cell death in breast cancer. J Med Chem. 2017;60(24):9990–10012. https://doi.org/10.1021/acs.jmedchem.7b00275.
Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Neuronal cell death. Physiol Rev. 2018;98(2):813–80. https://doi.org/10.1152/physrev.00011.2017.
Zhang Y, Zhan X, Xiong J, et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci Rep. 2018;8(1):8720. https://doi.org/10.1038/s41598-018-26978-1.
Kato A, Tatsumi Y, Yako H, et al. Recurrent short-term hypoglycemia and hyperglycemia induce apoptosis and oxidative stress via the ER stress response in immortalized adult mouse Schwann (IMS32) cells. Neurosci Res. 2019;147:26–32. https://doi.org/10.1016/j.neures.2018.11.004.
Sendoel A, Hengartner MO. Apoptotic cell death under hypoxia. Physiology. 2014;29(3):168–76. https://doi.org/10.1152/physiol.00016.2013.
Thornton C, Leaw B, Mallard C, Nair S, Jinnai M, Hagberg H. Cell death in the developing brain after hypoxia-ischemia. Front Cell Neurosci. 2017;11:248. https://doi.org/10.3389/fncel.2017.00248.
Narayanan KB, Ali M, Barclay BJ, et al. Disruptive environmental chemicals and cellular mechanisms that confer resistance to cell death. CARCIN. 2015;36(Suppl 1):S89–110. https://doi.org/10.1093/carcin/bgv032.
Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16(9):907–17. https://doi.org/10.1038/ni.3253.
Mandke P, Vasquez KM. Interactions of high mobility group box protein 1 (HMGB1) with nucleic acids: Implications in DNA repair and immune responses. DNA Repair. 2019;83:102701. https://doi.org/10.1016/j.dnarep.2019.102701.
He S-J, Cheng J, Feng X, Yu Y, Tian L, Huang Q. The dual role and therapeutic potential of high-mobility group box 1 in cancer. Oncotarget. 2017;8(38):64534–50. https://doi.org/10.18632/oncotarget.17885.
Tripathi A, Shrinet K, Kumar A. HMGB1 protein as a novel target for cancer. Toxicol Rep. 2019;6:253–61. https://doi.org/10.1016/j.toxrep.2019.03.002.
Calderwood SK, Gong J, Murshid A. Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front Immunol. 2016;7:159. https://doi.org/10.3389/fimmu.2016.00159.
Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. https://doi.org/10.1146/annurev.pathmechdis.3.121806.151456.
Tsan M-F. Toll-like receptors inflammation and cancer. Semin Cancer Biol. 2006;16(1):32–7. https://doi.org/10.1016/j.semcancer.2005.07.004.
Krysko DV, Leybaert L, Vandenabeele P, D’Herde K. Gap junctions and the propagation of cell survival and cell death signals. Apoptosis. 2005;10(3):459–69. https://doi.org/10.1007/s10495-005-1875-2.
Vanden Berghe T, Kalai M, Denecker G, Meeus A, Saelens X, Vandenabeele P. Necrosis is associated with IL-6 production but apoptosis is not. Cell Signal. 2006;18(3):328–35. https://doi.org/10.1016/j.cellsig.2005.05.003.
Ray CA, Pickup DJ. The mode of death of pig kidney cells infected with cowpox virus is governed by the expression of the crmA gene. Virology. 1996;217(1):384–91. https://doi.org/10.1006/viro.1996.0128.
Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15(2):135–47. https://doi.org/10.1038/nrm3737.
Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3 a novel apoptosis-inducing kinase. J Biol Chem. 1999;274(24):16871–5.
Dondelinger Y, Declercq W, Montessuit S, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7(4):971–81. https://doi.org/10.1016/j.celrep.2014.04.026.
Hildebrand JM, Tanzer MC, Lucet IS, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci USA. 2014;111(42):15072–7. https://doi.org/10.1073/pnas.1408987111.
Murphy JM, Czabotar PE, Hildebrand JM, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39(3):443–53. https://doi.org/10.1016/j.immuni.2013.06.018.
O’Donnell MA, Perez-Jimenez E, Oberst A, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13(12):1437–42. https://doi.org/10.1038/ncb2362.
Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14(11):727–36. https://doi.org/10.1038/nrm3683.
Vercammen D, Beyaert R, Denecker G, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187(9):1477–85. https://doi.org/10.1084/jem.187.9.1477.
Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8–independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–95. https://doi.org/10.1038/82732.
Cho Y, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–23.
Chen D, Yu J, Zhang L. Necroptosis: an alternative cell death program defending against cancer. Biochim Biophys Acta Rev Cancer. 2016;1865(2):228–36. https://doi.org/10.1016/j.bbcan.2016.03.003.
Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209–23. https://doi.org/10.1016/j.immuni.2013.02.003.
Petrie EJ, Sandow JJ, Lehmann WIL, et al. Viral MLKL homologs subvert necroptotic cell death by sequestering cellular RIPK3. Cell Rep. 2019;28(13):3309–19. https://doi.org/10.1016/j.celrep.2019.08.055.
Sedger LM, McDermott MF. TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants – past present and future. Cytokine Growth Factor Rev. 2014;25(4):453–72. https://doi.org/10.1016/j.cytogfr.2014.07.016.
Kalliolias GD, Ivashkiv LB. TNF biology pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12(1):49–62. https://doi.org/10.1038/nrrheum.2015.169.
Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–9. https://doi.org/10.1038/nchembio711.
Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–21. https://doi.org/10.1038/nchembio.83.
He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell. 2009;137(6):1100–11. https://doi.org/10.1016/j.cell.2009.05.021.
Zhang D-W, Shao J, Lin J, et al. RIP3 an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–6. https://doi.org/10.1126/science.1172308.
Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3(115):re4. https://doi.org/10.1126/scisignal.3115re4.
Kaiser WJ, Upton JW, Long AB, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–72. https://doi.org/10.1038/nature09857.
Oberst A, Dillon CP, Weinlich R, et al. Catalytic activity of the caspase-8–FLIPL complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–7. https://doi.org/10.1038/nature09852.
Zhang H, Zhou X, McQuade T, Li J, Chan FK-M, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471(7338):373–6. https://doi.org/10.1038/nature09878.
Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15(1):199. https://doi.org/10.1186/s12974-018-1235-0.
Pop C, Oberst A, Drag M, et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J. 2011;433(3):447–57. https://doi.org/10.1042/BJ20101738.
Xie T, Peng W, Yan C, Wu J, Gong X, Shi Y. Structural insights into RIP3-mediated necroptotic signaling. Cell Rep. 2013;5(1):70–8. https://doi.org/10.1016/j.celrep.2013.08.044.
Dondelinger Y, Aguileta MA, Goossens V, et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 2013;20(10):1381–92. https://doi.org/10.1038/cdd.2013.94.
Cai Z, Jitkaew S, Zhao J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16(1):55–65. https://doi.org/10.1038/ncb2883.
Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol. 2015;77:57–80. https://doi.org/10.1146/annurev-physiol-021014-071649.
Pryor PR, Luzio JP. Delivery of endocytosed membrane proteins to the lysosome. Biochim Biophys Acta Mol Cell Res. 2009;1793(4):615–24. https://doi.org/10.1016/j.bbamcr.2008.12.022.
Mindell JA. Lysosomal Acidification Mechanisms. Annu Rev Physiol. 2012;74(1):69–86. https://doi.org/10.1146/annurev-physiol-012110-142317.
Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10(9):623–35. https://doi.org/10.1038/nrm2745.
Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27(50):6434–51. https://doi.org/10.1038/onc.2008.310.
Mrschtik M, Ryan KM. Lysosomal proteins in cell death and autophagy. FEBS J. 2015;282(10):1858–70. https://doi.org/10.1111/febs.13253.
Appelqvist H, Sandin L, Björnström K, et al. Sensitivity to lysosome-dependent cell death is directly regulated by lysosomal cholesterol content. PLoS ONE. 2012;7(11):e50262. https://doi.org/10.1371/journal.pone.0050262.
Li W, Yuan X, Nordgren G, et al. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 2000;470(1):35–9. https://doi.org/10.1016/S0014-5793(00)01286-2.
Berg T, Gjøen T, Bakke O. Physiological functions of endosomal proteolysis. Biochem J. 1995;307(Pt 2):313–26. https://doi.org/10.1042/bj3070313.
Claus V, Jahraus A, Tjelle T, et al. Lysosomal enzyme trafficking between phagosomes, endosomes, and lysosomes in J774 macrophages: enrichment of cathepsin H in early endosomes. J Biol Chem. 1998;273(16):9842–51. https://doi.org/10.1074/jbc.273.16.9842.
Öllinger K, Brunk UT. Cellular injury induced by oxidative stress is mediated through lysosomal damage. Free Radic Biol Med. 1995;19(5):565–74. https://doi.org/10.1016/0891-5849(95)00062-3.
Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes in iron metabolism ageing and apoptosis. Histochem Cell Biol. 2008;129(4):389–406. https://doi.org/10.1007/s00418-008-0394-y.
Kurz T, Eaton JW, Brunk UT. Redox activity within the lysosomal compartment: implications for aging and apoptosis. Antioxid Redox Signal. 2010;13(4):511–23. https://doi.org/10.1089/ars.2009.3005.
Groth-Pedersen L, Ostenfeld MS, Høyer-Hansen M, Nylandsted J, Jäättelä M. Vincristine induces dramatic lysosomal changes and sensitizes cancer cells to lysosome-destabilizing siramesine. Cancer Res. 2007;67(5):2217–25. https://doi.org/10.1158/0008-5472.CAN-06-3520.
Quinn PJ. Is the distribution of -tocopherol in membranes consistent with its putative functions? Biochem (Moscow). 2004;69(1):58–66. https://doi.org/10.1023/B:BIRY.0000016352.88061.02.
Sahara S, Yamashima T. Calpain-mediated Hsp70.1 cleavage in hippocampal CA1 neuronal death. Biochem Biophys Res Commun. 2010;393(4):806–11. https://doi.org/10.1016/j.bbrc.2010.02.087.
Arnandis T, Ferrer-Vicens I, García-Trevijano ER, et al. Calpains mediate epithelial-cell death during mammary gland involution: mitochondria and lysosomal destabilization. Cell Death Differ. 2012;19(9):1536–48. https://doi.org/10.1038/cdd.2012.46.
Huang W-C, Lin Y-S, Chen C-L, Wang C-Y, Chiu W-H, Lin C-F. Glycogen synthase kinase-3β mediates endoplasmic reticulum stress-induced lysosomal apoptosis in leukemia. J Pharmacol Exp Ther. 2009;329(2):524–31. https://doi.org/10.1124/jpet.108.148122.
Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23(16):2881–90. https://doi.org/10.1038/sj.onc.1207512.
Bechara A, Barbosa CMV, Paredes-Gamero EJ, et al. Palladacycle (BPC) antitumour activity against resistant and metastatic cell lines: the relationship with cytosolic calcium mobilisation and cathepsin B activity. Eur J Med Chem. 2014;79:24–33. https://doi.org/10.1016/j.ejmech.2014.03.073.
Bové J, Martínez-Vicente M, Dehay B, et al. BAX channel activity mediates lysosomal disruption linked to Parkinson disease. Autophagy. 2014;10(5):889–900. https://doi.org/10.4161/auto.28286.
Castino R, Bellio N, Nicotra G, Follo C, Trincheri NF, Isidoro C. Cathepsin D-Bax death pathway in oxidative stressed neuroblastoma cells. Free Radic Biol Med. 2007;42(9):1305–16. https://doi.org/10.1016/j.freeradbiomed.2006.12.030.
Gómez-Sintes R, Ledesma MD, Boya P. Lysosomal cell death mechanisms in aging. Ageing Res Rev. 2016;32:150–68. https://doi.org/10.1016/j.arr.2016.02.009.
Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20(17):4629–33. https://doi.org/10.1093/emboj/20.17.4629.
Turk V, Stoka V, Vasiljeva O, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta Proteins Proteom. 2012;1824(1):68–88. https://doi.org/10.1016/j.bbapap.2011.10.002.
Vasiljeva O, Turk B. Dual contrasting roles of cysteine cathepsins in cancer progression: Apoptosis versus tumour invasion. Biochimie. 2008;90(2):380–6. https://doi.org/10.1016/j.biochi.2007.10.004.
Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833(12):3481–98. https://doi.org/10.1016/j.bbamcr.2013.06.026.
Kim Y-N, Koo KH, Sung JY, Yun U-J, Kim H. Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol. 2012;2012:1–11. https://doi.org/10.1155/2012/306879.
Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339(Pt 3):481–8.
Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9(5):701–6. https://doi.org/10.1016/s0955-0674(97)80124-x.
Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124(4):619–26. https://doi.org/10.1083/jcb.124.4.619.
Giancotti FG. Complexity and specificity of integrin signalling. Nat Cell Biol. 2000;2(1):E13-14. https://doi.org/10.1038/71397.
Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24(3):425–39. https://doi.org/10.1007/s10555-005-5134-3.
Brassard DL, Maxwell E, Malkowski M, Nagabhushan TL, Kumar CC, Armstrong L. Integrin alpha(v)beta(3)-mediated activation of apoptosis. Exp Cell Res. 1999;251(1):33–45. https://doi.org/10.1006/excr.1999.4559.
Matter ML, Zhang Z, Nordstedt C, Ruoslahti E. The alpha5beta1 integrin mediates elimination of amyloid-beta peptide and protects against apoptosis. J Cell Biol. 1998;141(4):1019–30. https://doi.org/10.1083/jcb.141.4.1019.
O’Brien V, Frisch SM, Juliano RL. Expression of the integrin alpha 5 subunit in HT29 colon carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp Cell Res. 1996;224(1):208–13. https://doi.org/10.1006/excr.1996.0130.
Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92(13):6161–5. https://doi.org/10.1073/pnas.92.13.6161.
Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134(3):793–9. https://doi.org/10.1083/jcb.134.3.793.
Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23(48):7906–9. https://doi.org/10.1038/sj.onc.1208160.
Böttcher RT, Lange A, Fässler R. How ILK and kindlins cooperate to orchestrate integrin signaling. Curr Opin Cell Biol. 2009;21(5):670–5. https://doi.org/10.1016/j.ceb.2009.05.008.
Khwaja A, Rodriguez-Viciana P, Wennström S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16(10):2783–93. https://doi.org/10.1093/emboj/16.10.2783.
Goldsmith ZG, Dhanasekaran DN. G Protein regulation of MAPK networks. Oncogene. 2007;26(22):3122–42. https://doi.org/10.1038/sj.onc.1210407.
Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76(11):1352–64. https://doi.org/10.1016/j.bcp.2008.07.023.
Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–62. https://doi.org/10.1016/s0955-0674(00)00251-9.
Gilmore AP. Anoikis. Cell Death Differ. 2005;12(Suppl 2):1473–7. https://doi.org/10.1038/sj.cdd.4401723.
Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226(2):380–93. https://doi.org/10.1002/path.3000.
Krishna S, Overholtzer M. Mechanisms and consequences of entosis. Cell Mol Life Sci. 2016;73(11–12):2379–86. https://doi.org/10.1007/s00018-016-2207-0.
Overholtzer M, Mailleux AA, Mouneimne G, et al. A Nonapoptotic Cell Death Process Entosis that Occurs by Cell-in-Cell Invasion. Cell. 2007;131(5):966–79. https://doi.org/10.1016/j.cell.2007.10.040.
Martins I, Raza SQ, Voisin L, et al. Entosis: The emerging face of non-cell-autonomous type IV programmed death. Biomed J. 2017;40(3):133–40. https://doi.org/10.1016/j.bj.2017.05.001.
Zeng C, Zeng B, Dong C, Liu J, Xing F. Rho-ROCK signaling mediates entotic cell death in tumor. Cell Death Discov. 2020;6(1):4. https://doi.org/10.1038/s41420-020-0238-7.
Wang M, Ning X, Chen A, et al. Impaired formation of homotypic cell-in-cell structures in human tumor cells lacking alpha-catenin expression. Sci Rep. 2015;5(1):12223. https://doi.org/10.1038/srep12223.
Sun Q, Cibas ES, Huang H, Hodgson L, Overholtzer M. Induction of entosis by epithelial cadherin expression. Cell Res. 2014;24(11):1288–98. https://doi.org/10.1038/cr.2014.137.
Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011;13(11):1335–43. https://doi.org/10.1038/ncb2363.
Wang S, Guo Z, Xia P, et al. Internalization of NK cells into tumor cells requires ezrin and leads to programmed cell-in-cell death. Cell Res. 2009;19(12):1350–62. https://doi.org/10.1038/cr.2009.114.
Cano CE, Sandí MJ, Hamidi T, et al. Homotypic cell cannibalism a cell-death process regulated by the nuclear protein 1 opposes to metastasis in pancreatic cancer. EMBO Mol Med. 2012;4(9):964–79. https://doi.org/10.1002/emmm.201201255.
Schwegler M, Wirsing AM, Schenker HM, et al. Prognostic value of homotypic cell internalization by nonprofessional phagocytic cancer cells. BioMed Res Int. 2015;2015:1–14. https://doi.org/10.1155/2015/359392.
Huang H, Chen A, Wang T, et al. Detecting cell-in-cell structures in human tumor samples by E-cadherin/CD68/CD45 triple staining. Oncotarget. 2015;6(24):20278–87. https://doi.org/10.18632/oncotarget.4275.
Li Y, Sun X, Dey SK. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep. 2015;11(3):358–65. https://doi.org/10.1016/j.celrep.2015.03.035.
Conrad M, Angeli JPF, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15(5):348–66. https://doi.org/10.1038/nrd.2015.6.
• Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. https://doi.org/10.1016/j.cell.2012.03.042. The reserachers identified the small molecule ferrostatin-1 as a potent inhibitor of ferroptosis in cancer cells.
Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis a new form of cell death and its relationships with tumourous diseases. J Cell Mol Med. 2017;21(4):648–57. https://doi.org/10.1111/jcmm.13008.
Feng H, Stockwell BR. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16(5):e2006203. https://doi.org/10.1371/journal.pbio.2006203.
Linkermann A, Skouta R, Himmerkus N, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA. 2014;111(47):16836–41. https://doi.org/10.1073/pnas.1415518111.
Kim SE, Zhang L, Ma K, et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11(11):977–85. https://doi.org/10.1038/nnano.2016.164.
Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–45. https://doi.org/10.1016/j.chembiol.2008.02.010.
Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–96. https://doi.org/10.1016/s1535-6108(03)00050-3.
Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–31. https://doi.org/10.1016/j.cell.2013.12.010.
Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12(7):497–503. https://doi.org/10.1038/nchembio.2079.
Hofmans S, Vanden Berghe T, Devisscher L, et al. Novel ferroptosis inhibitors with improved potency and ADME properties. J Med Chem. 2016;59(5):2041–53. https://doi.org/10.1021/acs.jmedchem.5b01641.
Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–91. https://doi.org/10.1038/ncb3064.
Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289–303. https://doi.org/10.1016/j.bbagen.2012.11.020.
Seiler A, Schneider M, Förster H, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8(3):237–48. https://doi.org/10.1016/j.cmet.2008.07.005.
Lu B, Chen XB, Ying MD, He QJ, Cao J, Yang B. The role of ferroptosis in cancer development and treatment response. Front Pharmacol. 2018;8:992. https://doi.org/10.3389/fphar.2017.00992.
Zheng D-W, Lei Q, Zhu J-Y, et al. Switching apoptosis to ferroptosis: metal-organic network for high-efficiency anticancer therapy. Nano Lett. 2017;17(1):284–91. https://doi.org/10.1021/acs.nanolett.6b04060.
Mou Y, Wang J, Wu J, et al. Ferroptosis a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34. https://doi.org/10.1186/s13045-019-0720-y.
Fearnhead HO, Vandenabeele P, Vanden BT. How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ. 2017;24(12):1991–8. https://doi.org/10.1038/cdd.2017.149.
Lee Y-S, Lee D-H, Choudry HA, Bartlett DL, Lee YJ. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol Cancer Res. 2018;16(7):1073–6. https://doi.org/10.1158/1541-7786.MCR-18-0055.
Ryter SW, Choi AMK. Cell death and repair in lung disease. In: McManus LM, Mitchell RN, editors. Pathobiology of Human Disease. Amsterdam: Elsevier; 2014. p. 2558–74. https://doi.org/10.1016/B978-0-12-386456-7.05302-8.
Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. https://doi.org/10.1038/nrmicro2070.
Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27(9):673–84. https://doi.org/10.1016/j.tcb.2017.05.005.
• Fernandes-Alnemri T, Wu J, Yu J-W, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14(9):1590–604. https://doi.org/10.1038/sj.cdd.4402194. The paper stated that the macrophage pyroptosis is mediated by a unique pyroptosome.
Tan Y, Chen Q, Li X, et al. Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res. 2021;40(1):153. https://doi.org/10.1186/s13046-021-01959-x.
Mathur A, Hayward JA, Man SM. Molecular mechanisms of inflammasome signaling. J Leukoc Biol. 2017;103(2):233–57. https://doi.org/10.1189/jlb.3MR0617-250R.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly regulation and signalling. Nat Rev Immunol. 2016;16(7):407–20. https://doi.org/10.1038/nri.2016.58.
Beere HM, Green DR. Immunologic repercussions of cell death. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, editors. Kelley and Firestein’s Textbook of Rheumatology. Amsterdam: Elsevier; 2017. p. 418–48. https://doi.org/10.1016/B978-0-323-31696-5.00028-0.
Li Y, Fan J, Ju D. Neurotoxicity concern about the brain targeting delivery systems. In: Gao H, Gao X, editors. Brain Targeted Drug Delivery System. Amsterdam: Elsevier; 2019. p. 377–408. https://doi.org/10.1016/B978-0-12-814001-7.00015-9.
Yi Y-S. Caspase-11 Non-canonical inflammasome: emerging activator and regulator of infection-mediated inflammatory responses. IJMS. 2020;21(8):2736. https://doi.org/10.3390/ijms21082736.
Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–25. https://doi.org/10.1111/j.1462-5822.2006.00751.x.
Elkon KB. Cell Survival and Death in Rheumatic Diseases. In: Saunders WB, editor. Kelley’s Textbook of Rheumatology. Amsterdam: Elsevier; 2013. p. 382–99. https://doi.org/10.1016/B978-1-4377-1738-9.00027-X.
Davis BK, Wen H, Ting JP-Y. The inflammasome NLRs in immunity inflammation and associated diseases. Annu Rev Immunol. 2011;29(1):707–35. https://doi.org/10.1146/annurev-immunol-031210-101405.
Vandanmagsar B, Youm Y-H, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–88. https://doi.org/10.1038/nm.2279.
Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci. 2000;97(26):14376–81. https://doi.org/10.1073/pnas.97.26.14376.
Valamanesh F, Torriglia A, Savoldelli M, et al. Glucocorticoids induce retinal toxicity through mechanisms mainly associated with paraptosis. Mol Vis. 2007;13:1746–57.
Torriglia A, Valamanesh F, Behar-Cohen F. On the retinal toxicity of intraocular glucocorticoids. Biochem Pharmacol. 2010;80(12):1878–86. https://doi.org/10.1016/j.bcp.2010.07.012.
Wei T, Kang Q, Ma B, Gao S, Li X, Liu Y. Activation of autophagy and paraptosis in retinal ganglion cells after retinal ischemia and reperfusion injury in rats. Exp Ther Med. 2015;9(2):476–82. https://doi.org/10.3892/etm.2014.2084.
Wang Y, Xu K, Zhang H, et al. Retinal ganglion cell death is triggered by paraptosis via reactive oxygen species production: A brief literature review presenting a novel hypothesis in glaucoma pathology. Mol Med Rep. 2014;10(3):1179–83. https://doi.org/10.3892/mmr.2014.2346.
Gong Y, Crawford JC, Heckmann BL, Green DR. To the edge of cell death and back. FEBS J. 2019;286(3):430–40. https://doi.org/10.1111/febs.14714.
Xie Y, Kang R, Tang D. Role of the beclin 1 network in the cross-regulation between autophagy and apoptosis. In: Hayat MA, editor. Autophagy: Cancer Other Pathologies Inflammation Immunity Infection and Aging. Amsterdam: Elsevier; 2016. p. 75–88. https://doi.org/10.1016/B978-0-12-802937-4.00002-8.
Radosevich J. Apoptosis and beyond: the many ways cells die. Hoboken: John Wiley & Sons Inc; 2018. https://doi.org/10.1002/9781119432463.
Khan S, Bhat AA. Nonenzymatic posttranslational protein modifications: mechanism and associated disease pathologies. In: Dar TA, Singh LR, editors. Protein Modificomics. Amsterdam: Elsevier; 2019. p. 229–80. https://doi.org/10.1016/B978-0-12-811913-6.00010-2.
Lyamzaev KG, Nepryakhina OK, Saprunova VB, et al. Novel mechanism of elimination of malfunctioning mitochondria (mitoptosis): Formation of mitoptotic bodies and extrusion of mitochondrial material from the cell. Biochim Biophys Acta Bioenerg. 2008;1777(7–8):817–25. https://doi.org/10.1016/j.bbabio.2008.03.027.
Jangamreddy JR, Los MJ. Mitoptosis a novel mitochondrial death mechanism leading predominantly to activation of autophagy. Hepat Mon. 2012;12(8):e6159. https://doi.org/10.5812/hepatmon.6159.
Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6(8):657–63. https://doi.org/10.1038/nrm1697.
Arnoult D, Rismanchi N, Grodet A, et al. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol. 2005;15(23):2112–8. https://doi.org/10.1016/j.cub.2005.10.041.
Hu T, Weng S, Tang W, et al. Overexpression of BIRC6 is a predictor of prognosis for colorectal cancer. PLoS ONE. 2015;10(5):e0125281. https://doi.org/10.1371/journal.pone.0125281.
Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in C elegans. Nature. 2001;412(6843):198–202. https://doi.org/10.1038/35084096.
Dong X, Lin D, Low C, et al. Elevated expression of BIRC6 protein in non–small-cell lung cancers is associated with cancer recurrence and chemoresistance. J Thorac Oncol. 2013;8(2):161–70. https://doi.org/10.1097/JTO.0b013e31827d5237.
Jiang SX, Lertvorachon J, Hou ST, et al. Chlortetracycline and demeclocycline inhibit calpains and protect mouse neurons against glutamate toxicity and cerebral ischemia. J Biol Chem. 2005;280(40):33811–8. https://doi.org/10.1074/jbc.M503113200.
Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359(5):473–81. https://doi.org/10.1056/NEJMoa071142.
Cudkowicz ME, Shefner JM, Simpson E, et al. Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. Muscle Nerve. 2008;38(1):837–44. https://doi.org/10.1002/mus.21059.
Weinreb O, Mandel S, Bar-Am O, et al. Multifunctional neuroprotective derivatives of rasagiline as anti-Alzheimer’s disease drugs. Neurotherapeutics. 2009;6(1):163–74. https://doi.org/10.1016/j.nurt.2008.10.030.
Patil AA, Bhor SA, Rheea WJ. Cell death in culture: Molecular mechanisms, detections, and inhibition strategies. Journal of Industrial and Engineering Chemistry. 2020;91:37–53. https://doi.org/10.1016/j.jiec.2020.08.009
Florey O, Krajcovic M, Sun Q, Overholtzer M. Entosis. Curr Biol. 2010;20(3):PR88–R89. https://doi.org/10.1016/j.cub.2009.11.020
Acknowledgements
The authors are very grateful to Deanship of Scientific Research (DSR) at the Majmaah University for their support and contribution to this study. Qamar Zia would like to thank Department of Medical Laboratories, Majmaah University, Majmaah, KSA, for providing the facility while Azfar Jamal acknowledge the College of Science, Department of Biology, Al-Zulfi, Majmaah University, Majmaah, KSA, for providing the support and facility to complete this work. Asim Azhar would like to acknowledge the director and principal of Aligarh College of Education, Aligarh for logistic support to carry out the research work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qamar Zia and Asim Azhar have equal contribution.
This article is part of the Topical Collection on Molecular Biology of Cell Death and Aging
Rights and permissions
About this article
Cite this article
Zia, Q., Azhar, A., Hassan, N. et al. Cell Death: a Molecular Perspective. Curr Mol Bio Rep 7, 41–66 (2021). https://doi.org/10.1007/s40610-021-00146-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-021-00146-3