Abstract
Summary
The patients’ persistence with osteoporosis treatments is low. This retrospective, multicenter survey showed that almost 30% of osteoporotic patients discontinued the treatment within the first 6 months and that those taking drinkable bisphosphonates were less likely to interrupt the therapy; instead, the use of generic bisphosphonates was associated to a more precocious interruption.
Purpose
Low persistence with osteoporosis medications is associated with higher fracture risk. This study aimed to assess the persistence to treatment with oral bisphosphonates among Italian osteoporotic patients under treatment for at least 6 months and to evaluate whether the different oral formulations of bisphosphonates may influence the interruption of the therapy.
Methods
723 consecutive osteoporotic patients, aged 50 years or over, referred as outpatients for a follow-up visit after receiving a prescription of an oral bisphosphonate for the first time for at least 6 months were enrolled in this retrospective, multicenter survey carried out under conditions of usual clinical practice. All the patients enrolled were submitted to a standardized interview.
Results
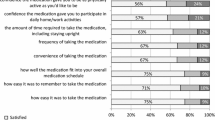
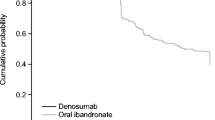
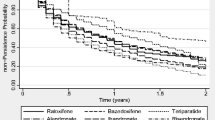
191 patients turned out to have discontinued treatment (28.7%), the more common causes for interruption being the adverse events (43.9%), fear of adverse events (23.3%) and perceived absence of efficacy of the treatment (15.8%). The osteoporotic patients taking drinkable bisphosphonate or on treatment with aromatase inhibitors or under the age of 70 years were less likely to interrupt the treatment. However, these associations were no longer significant when the pharmaceutical formulation (generic vs branded) was included into the multivariate logistic regression model.
Conclusion
This study suggests that the new drinkable formulations of bisphosphonates could be an interesting option able to reduce upper GI adverse events, thus increasing persistence; whereas the generic formulations of bisphosphonates were associated to a premature discontinuation.


Similar content being viewed by others
References
NIH consensus (2001) Development panel on osteoporosis prevention diagnosis and therapy. JAMA 285:785–795
Hernlund E, Svedbom A, Ivergård M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136
Kanis JA, McCloskey EV, Johansson H (2013) Scientific advisory board of the european society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO) and the committee of Scientific advisors of the international osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57
Hiligsmann M, Salas M, Hughes DA et al (2013) Interventions to improve osteoporosis medication adherence and persistence: a systematic review and literature appraisal by the ISPOR Medication Adherence and Persistence Special Interest Group. Osteoporos Int 24:2907–2918
Kothawala P, Badamgarav E, Ryu S et al (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82:1493–1501
Diaz-Perez A, Naylor KE (2017) Adherence working group of the international osteoporosis foundation and the European calcified tissue society. International osteoporosis foundation and European calcified tissue society working group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int 28:767–774
Kanis JA, Reginster JY, Kaufman JM et al (2012) A reappraisal of generic bisphosphonates in osteoporosis. Osteoporos Int 23:213–221
Siris ES, Selby PL, Saag KG et al (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122:S3–13
Patrick AR, Brookhart MA, Losina E et al (2010) The complex relation between bisphosphonate adherence and fracture reduction. J Clin Endocrinol Metab 95:3251–3259
Rabenda V, Mertens R, Fabri V et al (2008) Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. OsteoporosInt 19:811–818
Bianchi ML, Duca P, Vai S et al (2015) Improving adherence to and persistence with oral therapy of osteoporosis. Osteoporos Int 26:1629–1638
Gonnelli S, Caffarelli C, Rossi S et al (2016) How the knowledge of fracture risk might influence adherence to oral therapy of osteoporosis in Italy: the ADEOST study. Aging Clin Exp Res 28:459–468
Brandi ML, Black D (2013) A drinkable formulation of alendronate: potential to increase compliance and decrease upper GI irritation. Clin Cases Miner Bone Metab 10:187–190
Goldshtein I, Rouach V, Shamir-Stein N et al (2016) Role of side effects, physician involvement, and patient perception in non-adherence with oral bisphosphonates. Adv Ther 33:1374–1384
Modi A, Sajjan S, Michael Lewiecki E et al (2016) Relationship between gastrointestinal events and compliance with osteoporosis therapy: an administrative claims analysis of the US managed care population. Clin Ther 38:1074–1080
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
van Boven JF, de Boer PT, Postma MJ et al (2013) Persistence with osteoporosis medication among newly-treated osteoporotic patients. J Bone Miner Metab 31:562–570
Reyes C, Tebe C, Martinez-Laguna D et al (2017) One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int 28:2997–3004
Ström O, Landfeldt E (2012) The association between automatic generic substitution and treatment persistence with oral bisphosphonates. Osteoporos Int 23:2201–2209
Brown JP, Davison KS, Olszynski WP et al (2013) A critical review of brand and generic alendronate for the treatment of osteoporosis. Springer Plus 2:550
Walker AD, Adachi JD (2011) In vitro disintegration studies of weekly generic and branded risedronate sodium formulations available in Canada. Curr Med Res Opin 27:1749–1754
Grima DT, Papaioannou A, Airia P et al (2010) Adverse events, bone mineral density and discontinuation associated with generic alendronate among postmenopausal women previously tolerant to brand alendronate: a retrospective cohort study. BMC Muscoloskeletal Disorders 11:68–76
Invernizzi M, Cisari C, Carda S (2015) The potential impact of new effervescent alendronate formulation on compliance and persistence in osteoporosis treatment. Aging Clin Exp Res 27:107–113
Hodges LA, Connolly SM, Winter J et al (2012) Modulation of gastric pH by a soluble effervescent formulation: a possible mean of improving gastric tolerability of alendronate. Int J Pharm 432:57–62
Coaccioli S, Celi G, Crapa ME et al (2014) Alendronate soluble solution: a higher adherence rate in the treatment of osteoporosis. Clin Cases Miner Bone Metab 11:123–125
Schousboe JT (2013) Adherence with medications used to treat osteoporosis: behavioral insights. Curr Osteoporos Rep 11:21–29
Netelenbos JC, Geusens PP, Ypma G et al (2011) Adherence and profile of non-persistence in patients treated for osteoporosis-a large-scale, long-term retrospective study in The Netherlands. Osteoporos Int 22:1537–1546
Acknowledgements
We would like to thank Professor G. Cevenini for his advice in the revision of the statistical analysis.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, I declare no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration. Procedures performed in this study did not involve animals.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonnelli, S., Caffarelli, C., Letizia Mauro, G. et al. Retrospective evaluation of persistence in osteoporosis therapy with oral bisphosphonates in Italy: the TOBI study. Aging Clin Exp Res 31, 1541–1547 (2019). https://doi.org/10.1007/s40520-019-01205-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01205-7