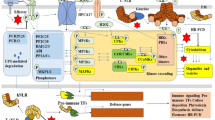
Abstract
A host-pathogen surpasses the plant defense machinery and successfully infects the plant to serve its needs. In contrast, a nonhost pathogen is restricted by plant immune responses. In this study, we deciphered the differential responses of Arabidopsis against the host-pathogen (Pseudomonas syringae pv. maculicola, Psm) and the nonhost pathogen (P. syringae pv. tabaci, Pst) infection. The Pst multiplication was restricted in Arabidopsis plant coinciding with the absence of any disease symptoms which was also associated with the increase in defense associated, pathogenesis related gene1 (PR1) expression and callose deposition. Host-pathogen infection, on the other hand, caused chlorotic symptoms with much less activation of the defense marker genes compared to the nonhost infection. Proline content was decreased in plants infected with Psm but not in case of Pst infection. Proline is a crucial plant metabolite, and osmolyte suggested to be involved in plant defense responses. However, the differential regulation of proline pathway under a susceptible or resistance response has not yet been unravelled. The expression profile of proline metabolism genes in a time course post-infection revealed drastic differences after Psm and Pst pathogen infection. The elevated expression of proline catabolic genes, AtProDH and AtP5CDH were noted under Psm infection. In contrast, plants infected with Pst showed upregulation of AtProDH but a decline in AtP5CDH transcripts along with an upregulation of the biosynthesis genes i.e. AtP5CS and AtP5CR. Our study shows that proline metabolism is tightly regulated under host and nonhost pathogen infection and impacts susceptibility and resistance of the plants, respectively.






Similar content being viewed by others
References
Ausubel, F. M. (2005). Are innate immune signaling pathways in plants and animals conserved? Nature Immunology,6(10), 973.
Ayliffe, M., & Sørensen, C. K. (2019). Plant nonhost resistance: Paradigms and new environments. Current Opinion in Plant Biology,50, 104–113.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil,39(1), 205–207.
Borsani, O., Zhu, J., Verslues, P. E., Sunkar, R., & Zhu, J. K. (2005). Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell,123(7), 1279–1291.
Cecchini, N. M., Monteoliva, M. I., & Alvarez, M. E. (2011). Proline dehydrogenase contributes to pathogen defense in Arabidopsis. Plant Physiology,155(4), 1947–1959.
Chen, X. Y., & Kim, J. Y. (2009). Callose synthesis in higher plants. Plant Signaling & Behavior,4(6), 489–492.
Choudhary, A., Gupta, A., Ramegowda, V., & Senthil-Kumar, M. (2017). Transcriptomic changes under combined drought and nonhost bacteria reveal novel and robust defenses in Arabidopsis thaliana. Environmental and Experimental Botany,139, 152–164.
Cui, H., Tsuda, K., & Parker, J. E. (2015). Effector-triggered immunity: from pathogen perception to robust defense. Annual Review of Plant Biology,66, 487–511.
Dangl, J. L., Horvath, D. M., & Staskawicz, B. J. (2013). Pivoting the plant immune system from dissection to deployment. Science,341(6147), 746–751.
Dangl, J. L., & Jones, J. D. (2001). Plant pathogens and integrated defence responses to infection. Nature,411(6839), 826.
Deuschle, K., Funck, D., Forlani, G., Stransky, H., Biehl, A., Leister, D., et al. (2004). The role of Δ1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell,16(12), 3413–3425.
Fabro, G., Kovács, I., Pavet, V., Szabados, L., & Alvarez, M. E. (2004). Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Molecular Plant-Microbe Interactions,17(4), 343–350.
Fatima, U., Bhorali, P., & Senthil-Kumar, M. (2019). Morpho-pathological and global transcriptomic analysis reveals the robust nonhost resistance responses in chickpea interaction with Alternaria brassicae. Molecular Plant-Microbe Interactions,32(12), 1598–1613.
Fernández-Bautista, N., Domínguez-Núñez, J. A., Moreno, M. C., & Berrocal-Lobo, M. (2016). Plant tissue trypan blue staining during phytopathogen infection. Bio-protocol,6(24), e2078.
Fonseca, J. P., & Mysore, K. S. (2019). Genes involved in nonhost disease resistance as a key to engineer durable resistance in crops. Plant Science,279, 108–116.
Gill, U. S., Lee, S., & Mysore, K. S. (2015). Host versus nonhost resistance: Distinct wars with similar arsenals. Phytopathology,105(5), 580–587.
Griffin, Eric A., & Carson, Walter P. (2015). The ecology and natural history of foliar bacteria with a focus on tropical forests and agroecosystems. Botanical Review,81(2), 105–149.
Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J., & Ahmad, A. (2012). Role of proline under changing environments: A review. Plant Signaling & Behavior,7(11), 1456–1466.
Hellmann, H., Funck, D., Rentsch, D., & Frommer, W. B. (2000). Hypersensitivity of an Arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiology,122(2), 357–368.
Jacob, C., Panchal, S., & Melotto, M. (2017). Surface inoculation and quantification of Pseudomonas syringae population in the Arabidopsis leaf apoplast. Bio-protocol,7(5), e2167.
Jones, J. D., & Dangl, J. L. (2006). The plant immune system. Nature,444(7117), 323.
Kang, L., Li, J., Zhao, T., Xiao, F., Tang, X., Thilmony, R., et al. (2003). Interplay of the Arabidopsis nonhost resistance gene NHO1 with bacterial virulence. Proceedings of the National Academy of Sciences USA,100(6), 3519–3524.
Lee, H. A., Lee, H. Y., Seo, E., Lee, J., Kim, S. B., Oh, S., et al. (2017). Current understandings of plant nonhost resistance. Molecular Plant-Microbe Interactions,30(1), 5–15.
Li, X., Lin, H., Zhang, W., Zou, Y., Zhang, J., Tang, X., et al. (2005). Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proceedings of the National Academy of Sciences USA,102(36), 12990–12995.
Lipka, U., Fuchs, R., & Lipka, V. (2008). Arabidopsis non-host resistance to powdery mildews. Current Opinion in Plant Biology,11(4), 404–411.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods,25(4), 402–408.
Lu, M., Tang, X., & Zhou, J. M. (2001). Arabidopsis NHO1 is required for general resistance against Pseudomonas bacteria. The Plant Cell,13(2), 437–447.
Maxwell, S. A., & Davis, G. E. (2000). Differential gene expression in p53-mediated apoptosis-resistant vs. apoptosis-sensitive tumor cell lines. Proceedings of the National Academy of Sciences USA,97(24), 13009–13014.
Melotto, M., Underwood, W., & He, S. Y. (2008). Role of stomata in plant innate immunity and foliar bacterial diseases. Annual review of Phytopathology,46, 101–122.
Mysore, K. S., & Ryu, C. M. (2004). Nonhost resistance: How much do we know? Trends in Plant Science,9(2), 97–104.
Nicks, R. E., & Marcel, T. C. (2009). Nonhost and basal resistance: How to explain specifity. New Phytologist,182(4), 817–828.
Nishimura, A., Nasuno, R., & Takagi, H. (2012). The proline metabolism intermediate Δ1-pyrroline-5-carboxylate directly inhibits the mitochondrial respiration in budding yeast. FEBS Letters,586(16), 2411–2416.
Nuernberger, T., & Lipka, V. (2005). Non-host resistance in plants: New insights into an old phenomenon. Molecular Plant Pathology,6(3), 335–345.
Qamar, A., Mysore, K., & Senthil-Kumar, M. (2015). Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Frontiers in Plant Science,6, 503.
Rejeb, I., Pastor, V., & Mauch-Mani, B. (2014). Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants,3(4), 458–475.
Schenk, S. T., & Schikora, A. (2015). Staining of callose depositions in root and leaf tissues. Bio-protocol,5(6), e1429.
Senthil-Kumar, M., & Mysore, K. S. (2012). Ornithine-delta-aminotransferase and proline dehydrogenase genes play a role in non-host disease resistance by regulating pyrroline-5-carboxylate metabolism-induced hypersensitive response. Plant, Cell and Environment,35(7), 1329–1343.
Senthil-Kumar, M., & Mysore, K. S. (2013). Nonhost resistance against bacterial pathogens: Retrospectives and prospects. Annual review of Phytopathology,51, 407–427.
Speth, E. B., Lee, Y. N., & He, S. Y. (2007). Pathogen virulence factors as molecular probes of basic plant cellular functions. Current Opinion in Plant Biology,10(6), 580–586.
Thordal-Christensen, H. (2003). Fresh insights into processes of nonhost resistance. Current Opinion in Plant Biology,6(4), 351–357.
Tripathy, J. N., Zhang, J., Robin, S., Nguyen, T. T., & Nguyen, H. T. (2000). QTLs for cell-membrane stability mapped in rice (Oryza sativa L.) under drought stress. Theoretical and Applied Genetics,100(8), 1197–1202.
Verslues, P. E., & Sharma, S. (2010). Proline metabolism and its implications for plant–environment interaction. The Arabidopsis Book/American Society of Plant Biologists,8, e0140.
Yu, X., Feng, B., He, P., & Shan, L. (2017). From chaos to harmony: Responses and signaling upon microbial pattern recognition. Annual review of Phytopathology,55, 109–137.
Zhao, Y., Damicone, J. P., Demezas, D. H., Rangaswamy, V., & Bender, C. L. (2000). Bacterial leaf spot of leafy crucifers in Oklahoma caused by Pseudomonas syringae pv. maculicola. Plant Disease,84(9), 1015–1020.
Acknowledgements
Aarzoo Qamar acknowledges UGC (23/12/2012(ii) EU-V, 17/7/2013) and NIPGR for providing funding. Authors thank Dr. Aarti Gupta for critical reading and editing of the manuscript and Dr. Mahesh Patil for internal reviewing of the manuscript. Authors acknowledge Mr. Rahim Tarafdar for plant care and Mr. Sunder Solanki for technical help in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qamar, A., Senthil-Kumar, M. Arabidopsis exhibits differential response in basal immunity and proline metabolism during defense against host and nonhost pathogen infection. Plant Physiol. Rep. 24, 496–506 (2019). https://doi.org/10.1007/s40502-019-00480-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-019-00480-w