Abstract
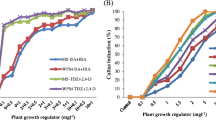
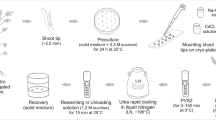
The current study reports on in vitro propagation and conservation of critically endangered medicinal plant Swertia chirayita. The sterilized explants (leaves) were cultured on Murashige and Skoog’s (MS) medium supplemented with 0.1 mg/L naphthalene acetic acid (NAA) and 3.0 mg/L benzyl adenine (BA) for callus induction, the same medium was used for shoot regeneration and for direct shoot regeneration from in vitro leaves. For in vitro multiplication shoots were cultured on MS medium supplemented with 2.5 mg/L benzyl adenine and 0.1 mg/L Kinetin (Kn). MS half strength medium supplemented with 400 mg/L activated charcoal and 0.1 mg/L naphthalene acetic acid showed 80.30% root induction from in vitro grown shoots. The in vitro raised plantlets were successfully acclimatized. All the regenerated plantlets appeared normal with 70–80% survival rate, while In vitro conservation was carried out by using two different approaches namely slow growth by changing media composition (sucrose and abscisic acid) at low temperature and cryopreservation following vitrification. With increase in concentration of sucrose and ABA decrease in growth of in vitro shoots was observed. At low temperature the in vitro shoots incubated at 4 and 10 °C both showed 100% retrieval. During studies the vitrified shoot gave retrieval of 42.33% when pre-cooled at 4 °C while only 22.77% vitrified shoots were retrieved from those pre-cooled at 10 °C.




Similar content being viewed by others
References
Balaraju, K., Agastian, P., & Ignacimuthu, S. (2009). Micropropagation of Swertia chirata Buch. Hams. ex Wall: A critically endangered medicinal herb. Acta Physiologiae Plantarum, 31(3), 487–494.
Bisht, S. S., & Bisht, N. S. (2008). Callus induction studies in different explants of Swertia angustifolia (Buch-Ham). Plant Archives, 8(2), 713–716.
Chaudhuri, R. K., Pal, A., & Jha, T. B. (2008). Conservation of Swertia chirata through direct shoot multiplication from leaf explants. Plant Biotechnology Report, 2, 213–221.
Dafadar, A., & Jha, T. B. (2011). In vitro propagation and conservation of Swertia bimaculata Hook.f. and Thomas. Indian Journal of Biotechnology, 11, 295–299.
Ding, W., Song, L., Wang, X., & Bi, Y. (2010). Effect of ABA and heat stress tolerance in the calli form two ecotypes of phragmits communis. Biologia Plantaerum, 54, 67–613.
Engelmann, F. (2011). Use of biotechnologies for the conservation of plant biodiversity. In vitro Cellular and Development Biology—Plant, 47, 5–16.
Fortes, G., Rde, L., & Pereira, J. E. S. (2001). Effect of low temperature and growth retardants on in vitro conservation of asparagus. Revista Cientifica Rural, 6(2), 181–186.
Goncalves, S., & Romano, A. (2007). In vitro minimum growth for conservation of Drosophyllum lusitanicum. Biologia Plantarum, 51, 795–798.
Gopal, J., Anjali, C., & Debabrata, S. (2003). Use of microtubers for slow growth in vitro conservation of potato. Journal of the Indian Potato Association, 30(1/2), 35–36.
Gopal, J., Chamail, A., & Sarkar, D. (2004). In vitro production of microtubers for conservation of potato germplasm: Effect of genotype, abscisic acid, and sucrose. In vitro Cellular and Developmental Biology—Plant, 40, 485–490.
Halmagye, A., & Pinkar, I. (2006). Cryopreservation of Rosa shoot tips: Importance of precultured conditions. Acta Horticulturae, 725, 351–356.
He, T., Xu, J., Yang, L., & Wang, H. (2012). An efficient method for plant regeneration from calli of Swertia mussotii, an endangered medicinal herb. American Journal of Plant Sciences, 3(7), 904–908.
Joshi, P., & Dhawan, V. (2005). Swertia chirayita—An overview. Current Science, 89, 635–640.
Joshi, P., & Dhawan, V. (2007). Axillary multiplication of Swertia chirayita: A critically endangered medicinal herb of temperate Himalayas. In Vitro Cellular and Developmental Biology Plant, 43(6), 631–638.
Kashyap, A. (2009). Studies on in vitro propagation and conservation of Inula racemosa. In Hook. F (Ed.). Ph. D. Thesis. Dr. Y S Parmar University of Horticulture and Forestry, 158 p.
Kavimani, S., & Manisenthlkumar, K. T. (2011). Effect of methanolic extract of Enicostemma littorale on Dalton’s ascitic lymphoma. Journal of Ethnopharmacology, 71, 349–352.
Kumar, V., & Chandra, S. (2013). Efficient regeneration and antioxidant activity of the endangered species Swertia chirayita. International Journal of Pharma and Bio Sciences, 4(4), 823–833.
Kumar, A., Guleria, S., Mehta, P., Walia, A., Chauhan, A., & Shirkot, C. K. (2015). Plant growth-promoting traits of phosphate solubilizing bacteria isolated from Hippophae rhamnoides L. (Sea-buckthorn) growing in cold desert Trans-Himalayan Lahul and Spiti regions of India. Acta Physiologiae Plantarum, 37(3), 1–12.
Lata, H., Moraes, R. M., Bertoni, B., & Pereira, A. M. S. (2010). In vitro germplasm conservation of Podophyllum peltatum L. under slow growth conditions. In vitro Cellular and Developmental Biology—Plant, 46, 22–27.
Manoj, K., Rai, N. S., Shekhowat, H. K., & Gupta, A. (2011). The role of ABA in plant tissue culture: A review of recent progress. Plant Cell, Tissue and Organ Culture, 106, 179–190.
Maubmann, V., Serek, M., & Winkelmann, T. (2006). Cryopreservation of embryogenic suspension of cultures of Cyclamen persicum Mill. Acta Horticulturae, 725, 391–396.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantareum, 15, 473–497.
Padhan, J. K., Kumar, V., Sood, H., Singh, T. R., & Chauhan, R. S. (2015). Contents of therapeutic metabolites in Swertia chirayita correlate with the expression profiles of multiple genes in corresponding biosynthesis pathways. Phytochemistry, 116, 38–47.
Pal, D., Sur, S., Mandal, S., Das, A., Roy, A., Das, S., et al. (2012). Prevention of liver carcinogenesis by amarogentin through modulation of G1/S cell cycle check point and induction of apoptosis. Carcinogenesis, 33, 2424–2431.
Pant, M., Bisht, P., & Gusain, M. P. (2009). De novo shoot organogenesis from cultured root explants of Swertia chirata Buch.-Ham.ex Wall: An endangered medicinal plant. African Journal of Biotechnology, 11(29), 7408–7416.
Pant, M., Bisht, P., & Gusain, M. P. (2011). In vitro propagation through root derived callus culture of Swertia chirata Buch. Ham. ex Wall. African Journal of Biotechnology, 11(29), 7408–7416.
Patil, M. S., Adiga, J. D., Reddy, B. S., Kulkarni, B. S., Hegde, L., & Mulge, R. (2004). Effect of slow growth strategies for in vitro conservation of gladiolus. Karnataka Journal of Horticulture, 1(1), 39–43.
Phoboo, S., Pinto, M. D. S., Barbosa, A. C. L., Sarkar, D., Bhowmik, P. C., Jha, P. K., et al. (2013). Phenolic-linked biochemical rationale for the anti-diabetic properties of Swertia chirayita (Roxb. ex Flem.) Karst. Phytotherapy Research, 27, 227–235.
Pruski, K., Kozai, T., Lewis, T., Astakie, T., & Novak, J. (2000). Sucrose and light effects on in vitro cultures of potato, chokecherry and Saskatoon berry during low temperature storage. Plant Cell, Tissue and Organ Culture, 63, 215–221.
Shailja, (2017). A mini review on in vitro propagation of Swertia chirayita an endangered medicinal plant. Bioscience Biotechnology Research Communication, 10(1), 8–12.
Shibli, R. D., Shatnawi, M. A., Subaih, W. S., & Ajlouni, M. M. (2006). In vitro conservation and cryopreservation of plant genetic resources: A review. World Journal of Agricultural Sciences, 2, 372–382.
Soni, M., & Kaur, R. (2013). Rapid in in vitro propagation, conservation and analysis of genetic stability of Viola pilosa. Physiology and Molecular Biology Plants. doi:10.1007/s1298-013-0200-8.
Turner, S. R., & Singha, S. (1990). Vitrification of crabapple, pear, and geum on gellan gum solidified culture medium. Hortscience, 25(12), 1648–1649.
Vaidya, H., Goyal, R. K., & Cheema, S. K. (2013). Anti-diabetic activity of swertiamarin is due to an active metabolite, gentianine, that upregulates PPAR-c gene expression in 3T3-L1 cells. Phytotherapy Research, 27, 624–627.
Wang, L., An, L., Hu, Y., Ping, W., Lixin, W., & Li, Y. (2009). Influence of phytohormones and medium on the shoot regeneration from leaf of Swertia chirata Buch. Ham. ex Wall in vitro. African Journal of Biotechnology, 8(11), 2513–2517.
Wang, J., Zhao, C., Liu, C., Xia, G., & Xiang, F. (2011). Introgression of Swertia mussotii gene into Bupleurum scorzonerifolium via somatic hybridization. BMC Plant Biology, 11, 71.
Wawrosch, C., Maskay, N., & Koop, B. (1999). Micropropagation of the threatened Nepalese medicinal plant Swertia chirata Buch-Ham. Ex Wall. Plant Cell Report, 18(12), 997–1001.
Acknowledgement
We thanks Dr Y S Parmar Univ. of Horticulture and Forestry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest among them.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shailja, Kanwar, K., Soni, M. et al. In vitro propagation and conservation of an endangered high value medicinal herb Swertia chirayita of temperate Himalayas. Ind J Plant Physiol. 22, 247–257 (2017). https://doi.org/10.1007/s40502-017-0294-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-017-0294-z