Abstract
Background
Antimicrobial resistance is a growing public health concern. There is a global need to estimate the population-level value of developing new antimicrobials and to ensure the effective use of existing antimicrobials as strategies to counteract antimicrobial resistance. To this aim, population-level value criteria need to be considered alongside conventional value measures.
Objective
The objective of this study was to develop a novel modelling approach to estimate the value of new antimicrobials, considering the transmission, diversity and enablement elements of STEDI value.
Methods
We developed a population-based mathematical model for the assessment of antimicrobial value considering both prophylactic use of antimicrobials and the treatment of selected serious hospital-acquired infections in hospitals in the USA at a population level. Large-scale clinical and population healthcare data were used to inform a modelling-based analysis assessing the impact of introducing a new antimicrobial compared with continuing with no new antimicrobial, accounting for the transmission, diversity and enablement value of antimicrobial agents.
Results
Over a 10-year period, the addition of a new antimicrobial as part of an antimicrobial stewardship strategy in the USA was estimated to result in a proportional reduction of 9.03% in projected antimicrobial resistance levels. This yielded an estimated reduction of $64.3 million in hospitalization costs and a gain of over 153,000 quality-adjusted life-years at an economic value of over $15.4 billion over 10 years. Considering input uncertainty, the estimate of monetary benefit ranged from $11.1 to $21.4 billion.
Conclusions
The use of a new antimicrobial for treatment and prophylactic indications yields considerable clinical and economic benefits including transmission diversity and enablement value. These findings may provide decision makers with important evidence to support investment in new antimicrobials and antimicrobial stewardship policy that address the patient, population and system burden associated with antimicrobial resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first study to demonstrate the population-level clinical and economic value of a new antimicrobial in the USA, when considering transmission, diversity and enablement value, estimated at over $15.4 billion over 10 years. |
These findings can inform policy decisions to support investment in the research and development of novel antimicrobials in the USA. |
1 Introduction
The global rise in antimicrobial resistance (AMR) is a significant threat to public health. Globally, AMR is a leading cause of death; it is estimated that 1.27 million deaths were attributable to AMR in 2019 [1]. In the USA alone, over 2.8 million antimicrobial-resistant infections are thought to occur annually, leading to more than 35,000 deaths [2]. The dwindling pipeline of effective antimicrobials and their inappropriate use complicate the treatment of several conditions, including secondary bacterial infections in patients with coronavirus disease 2019, and jeopardise the ability to safely provide cancer chemotherapy, transplants and other surgeries [3, 4]. In the setting of antimicrobial prophylaxis, it has been estimated that up to 50.9% of pathogens causing surgical-site infections and 26.8% of pathogens causing infections after chemotherapy are resistant to standard antimicrobials used in the USA [5].
It is not just the public health burden that is of concern; the global economic damage caused by AMR is also substantial. The World Bank estimates that, by 2050, the economic impact of AMR could be similar to that of the 2008 financial crisis, with potential annual losses to global gross domestic product of 3.8% [6]. Despite this, antimicrobial innovation is lacking, with research and development (R&D) costs exceeding expected revenues [7,8,9]. This has led to bankruptcy in some smaller companies and some large companies abandoning the antimicrobial market [4, 10, 11]. Using traditional reimbursement models, revenue is determined by the number of sales and the treatment price; however, antimicrobial stewardship (AMS) schemes aim to limit the consumption of antimicrobials, restricting sales and therefore, returns. In addition, conventional health technology assessment methods employ measures that define value, reflecting patient-level outcomes including unmet needs, health benefits, cost offsets (e.g. reduced hospital stays) and productivity benefits (e.g. faster return to work). This approach is appropriate in the evaluation of non-communicable diseases; however, antimicrobials are associated with a number of additional value criteria that reflect the population-level impact of antimicrobials on AMR, referred to as “STEDI” (spectrum, transmission, enablement, diversity and insurance) described in Table 1 [11,12,13]. Novel access and reimbursement mechanisms have been proposed to reflect the broader multi-stakeholder value associated with new antimicrobials, and to encourage investment by reducing the financial risk in antimicrobial R&D. However, despite a broad consensus, the application of STEDI concepts to estimate the value of antimicrobials is rarely implemented in practice in health technology assessments.
Previous research efforts have made progress towards capturing these STEDI value elements, outlining a modelling approach that considers the transmission and diversity components [14]. However, spectrum, enablement, and insurance value remain unaccounted for and therefore this approach produces a conservative estimate of antimicrobial value. There are several obstacles to developing a comprehensive model of antimicrobial value. The most significant limitation is the availability of evidence; previous attempts were unable to capture the spectrum value because of the limited availability of data to characterise the relationship between antimicrobial spectrum (narrow vs broad) and resistance development [14]. A methodological review of economic studies of AMR highlighted that current approaches failed to include the benefits of improved prophylaxis outcomes, and therefore to consider the enablement value of antimicrobials [15].
Informed public health policy requires an integrated assessment of the total value associated with AMR and AMS strategies, from the perspective of health systems, patients and society. In this study, we aim to build upon previous research to develop a novel modelling approach estimating the value of new antimicrobials using data from the USA, considering the transmission, diversity and enablement elements of STEDI value; to generate evidence to support informed antimicrobial policy and funding decisions from the US perspective. The approach presented here may be adapted to other countries generating evidence valuable to other healthcare systems, when addressing the challenges of AMR.
2 Methods
2.1 Model Overview
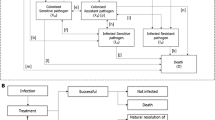
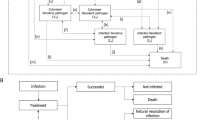
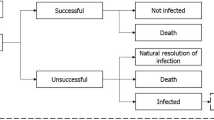
A population-based mathematical model was developed, using Microsoft Excel, to assess the value of introducing a new antimicrobial in treating hospital-acquired infections (HAIs) (treatment setting) and the prophylactic use to prevent infection during surgery and chemotherapy (prophylactic setting). The effectiveness of antimicrobials to treat and prevent infection is defined by the relationship between antimicrobial consumption (exposure) and AMR. The introduction of a new antimicrobial and the appropriate use of existing antimicrobials are considered in the context of the AMS principle that increased treatment diversity reduces selection pressure on existing antimicrobial treatments, and thus is considered to reduce the projected gain in population-level resistance (i.e. the mean resistance level across all pathogens and treatments). Reducing resistance gain leads to improved population-level effectiveness in both the treatment and prophylactic settings, when compared to without the introduction of a new antimicrobial. The value of introducing a new antimicrobial is captured in the model by improvements in population-level outcomes including reduced rates of infection, deaths, and hospital activity in both treatment and prophylactic settings. Figure 1 outlines the relationships between variables within the model, in a causal loop diagram, additional factors beyond the scope of the current analysis were also included to provide the wider context.
Causal loop diagram demonstrating the interactions between modelled variables (yellow) to generate modelled outcomes (blue) within the hospital setting (the impact of antimicrobial use in the community and environmental settings are not covered within this diagram). Green arrows show the impact of introducing a new antimicrobial on each of the variables and the clinical and economic outcomes. The shaded areas show how the variables have been used to estimate transmission, diversity and enablement value. A new product increases antimicrobial diversity, reducing selection pressure and antimicrobial resistance, reduced antimicrobial resistance increases antimicrobial efficacy in the treatment and prophylactic setting, leading to fewer surgical- and chemotherapy-related infections and more effective hospital-acquired infection treatment, and reduced transmission of resistant infections. Variables and outcomes in white are not measured within the current model but have important influences on the measured variables. R&D research and development
A deterministic mathematical model was developed building upon previous AMR modelling studies [5, 14]. This model has two major components, outlined in a model schematic (Fig. S1 of the Electronic Supplementary Material [ESM]). The first component captures the effects of a new antimicrobial being introduced compared to no new antimicrobial in the treatment setting considering HAIs (capturing transmission and diversity value). The second component assesses the effects of a relative reduction in population-level AMR (derived from the impact of introducing a new antimicrobial compared with continuing without the addition of a new antimicrobial on resistance gain captured in the treatment setting) by means of a change in prophylactic efficacy in the prophylactic setting (capturing enablement value). A model analysis was undertaken from the healthcare system perspective to estimate the relationship between changes in AMR, antimicrobial efficacy and outcomes at a population level.
2.2 Treatment Setting
The treatment component is adapted from a published and validated dynamic model and has been described previously [14], in short, this model considered the impact of a new antimicrobial on the transmission of infection and resistance in an infectious environment to estimate the health economic value. The treatment setting in the current study uses regression equations to summarise the transmission dynamics and relationships for drivers of model outputs from the previously published model [14]. This approach was taken to reduce the model complexity and data requirements. The regression equations were derived by running over 1 million simulations, varying inputs for the population, baseline resistance, treatment strategy, treatment duration and treatment efficacy in the previously published model [14]. Linear regression models were applied to time on treatment, the number resistant to each treatment, and death, and were derived separately based on if two or three lines of treatment were modelled, which were then used to estimate model outcomes presented as a pooled estimate by pathogen and indication. Outputs of the linear regression models are presented in the ESM.
Inputs relating to treatment efficacy, baseline resistance, hospitalisation costs and length of stay, life expectancy and health state utilities (Table S2 of the ESM) are applied to the regression equations to estimate health economic outcomes for treating the modelled infections. Life-year (LY) and quality-adjusted life-year (QALY) outcomes are linked to the mortality equation, where utility values related to infected and non-infected patients and life expectancy post-successful treatment are applied. Cost outcomes are linked to the mortality and time on treatment equations where daily hospitalisation costs are applied (Fig. S1 of the ESM). An internal validation compared model outcomes against outcomes from the previously published model, which has been extensively validated against external data [14]. The disease transmission component of the published model, on which the regression equations were based, was calibrated to estimate resistance change and indication-specific infection incidence using UK data [14]; these dynamics were validated using historic resistance data for hospital-acquired infections in the USA reported from the National Healthcare Safety Network at the Centers for Disease Control and Prevention. The model was parameterised with resistance data from 2011 and the predicted outcomes for resistance were compared against reported data for 2012, 2013 and 2014 [16]. These validation exercises are described in Figs. S2 and S3 of the ESM.
2.3 Prophylactic Setting
In the prophylactic component of the model, outcomes are assessed in the context of preventing infection during surgery and chemotherapy. The change in prophylactic antimicrobial efficacy was estimated at a population level, dependent on antimicrobial exposure in the treatment setting. Antimicrobial exposure is estimated within a treatment pathway under two scenarios using projected population-level resistance, from the treatment setting, for the two-line treatment strategy (representing continuing with no new antimicrobial) and the three-line treatment strategy (representing the addition of a new antimicrobial to treatment options). A change in antimicrobial efficacy is estimated from antimicrobial exposure using the following equation.
The change in population-level resistance estimated in the treatment setting is assumed to be reflected in the prophylactic setting and therefore, the proportional change in prophylactic antimicrobial efficacy was assumed to be equivalent to the estimated change in antimicrobial efficacy. The prophylactic component utilises research conducted by Teillant et al. [5] to link the improvement in prophylactic efficacy to outcomes. The authors conducted a literature review and meta-analysis of randomised controlled trials assessing the efficacy of antimicrobial prophylaxis treatment on outcomes of surgical procedures and immunosuppressing chemotherapy. Using the absolute risk reduction in infection (ARRi) rates between antimicrobial prophylaxis and control groups, they were able to estimate the number of infections and deaths avoided across the annual number of procedures (Ni), given changes in prophylactic antimicrobial efficacy, using the following equations, where i denotes the procedure:
Using the methodology described by the authors, the prophylactic component estimated the number of infections and infection-related deaths for each of the ten most common surgical procedures and cancer chemotherapies in the USA, by applying the estimated percentage improvements in prophylactic efficacy to published procedure-specific rates of infection and mortality from infection [5]. Procedure-specific utilities and life expectancies were applied to infections and deaths avoided to calculate QALYs gained in the prophylactic setting.
2.4 Model Inputs
The treatment component was populated with data from the US setting (model inputs detailed in Table S2 of the ESM), where outcomes were evaluated over a disease transmission horizon of 10 years based on an average of 19,396 HAIs per annum. These consisted of complicated urinary tract infections, complicated intra-abdominal infections and hospital acquired/ventilator-associated pneumonia caused by the three most common gram-negative pathogens (Escherichia coli, Klebsiella spp. and Pseudomonas aeruginosa) in US HAIs [17]. Whilst the model assesses outcomes for all indications of interest, the estimated annual infection incidence was informed by data from the 2019 National and State Healthcare-associated Infections Progress Report [18], which lacked data on complicated intra-abdominal infections. Therefore, the overall number of annual infections excludes complicated intra-abdominal infections. Current therapy was represented by piperacillin/tazobactam (first line) and meropenem (second line) for each of the three pathogens and indications of interest, informed by clinical guidance and expert opinion [19,20,21]. Antimicrobial resistance estimates were sourced from the most recent national summary of AMR (2015–17) from the National Healthcare Safety Network, considering all reported HAIs [17]. Antimicrobial resistance levels for carbapenems were used as a proxy for meropenem, and where data were not available for piperacillin/tazobactam, AMR rates for extended-spectrum cephalosporin were used as a proxy for E. coli and Klebsiella spp.; E. coli and Klebsiella spp. strains resistant to extended-spectrum cephalosporins are usually producers of extended-spectrum beta-lactamases and therefore will be resistant against piperacillin/tazobactam [22, 23]. Treatment efficacy in patients with no resistance to treatment for piperacillin/tazobactam and meropenem were obtained from randomised controlled trials and were estimated as a weighted average across the modelled pathogen and indications. As this analysis considered the addition of a hypothetical novel antimicrobial, its efficacy was assumed and the value reflected a moderate improvement on the comparator treatments. Hospitalisation costs were taken from the 2019 Centers for Medicare and Medicaid Services costs report [24].
The prophylactic component included the ten most common surgical procedures and immunosuppressing cancer chemotherapies in the USA as identified by Teillant et al. [5] The annual number of procedures are presented in Table S3 of the ESM.
The absolute risk reductions for infection/serious infection incidence and mortality for each procedure form the basis for the evaluation of annual infections and deaths avoided and are detailed in Tables S3–5 of the ESM. Procedure-specific utility decrements for infection, post-procedure life expectancy and post-procedure utility values are presented in Tables S6–9 of the ESM.
2.5 Data Sources
For national data on HAI incidence, the current model draws on the 2019 National and State Healthcare-Associated Infections Progress Report produced by the Centers for Disease Control and Prevention National Healthcare Safety Network [18]. This source reports HAI data from almost 38,000 healthcare facilities across all 50 states in the USA. Evidence on national-level data on AMR for HAIs was also sourced from the NHSN reported by the Centers for Disease Control and Prevention [17]. Estimates of the incidence of surgical and chemotherapy treatments were sourced from Teillant et al. [5] and were based on the Centers for Disease Control and Prevention National Hospital Discharge Survey, the National Cancer Data Base or from the published scientific literature.
2.6 Model Outputs
The treatment setting of the model estimates the population-level value to patients and the healthcare system of achieving more effective treatment of infections through reductions in AMR in terms of cost offsets, LYs and QALYs gained [1]. The prophylactic setting was used to estimate the value to patients of reduced infections/deaths associated with more effective prophylactic antimicrobials, as determined by changes in AMR [2] Total value ([1] + [2]) was calculated based on the frequency of antimicrobial use in the treatment setting and in the prophylactic setting.
To quantify the economic outcomes associated with a reduction in AMR levels, we calculated the monetary benefit (MB), which is defined as follows:
We aimed to quantify the overall value to healthcare systems a new antimicrobial would provide; therefore, only hospitalization costs were considered. This is aligned with the NHS England/NICE pilot scheme approach, where treatment costs are excluded, to estimate the population-level economic value relevant to investment decisions and not as an assessment of cost effectiveness [29].
2.7 Model Analysis
2.7.1 Base Case
We estimated the potential population value of antimicrobials used in prophylactic and treatment-based settings by comparing model outputs under the intervention (introduction of a hypothetical new antimicrobial, within an AMS strategy) to those obtained under current practice (without the introduction of a new antimicrobial). The current practice consists of two lines of treatment (piperacillin/tazobactam first line followed by meropenem second line). The analysis assumed a willingness-to-pay (WTP) threshold of $100,000/QALY and applied a discount rate of 3.0% per year [25]
2.7.2 Sensitivity Analysis
The sensitivity of the model to input estimates was tested deterministically in a series of one-way sensitivity analyses. Key model inputs relating to treatment efficacy, baseline resistance, utilities, and hospitalisation input listed in Tables S2 and S3–9 of the ESM were adjusted by ± 20% (proportions were maintained between 0 and 100%) and the impact was assessed according to the combined MB across both the treatment and prophylactic settings combined. Scenarios were also explored using a WTP threshold of $50,000 and $200,000.
To test the impact of input uncertainty on the model outcomes, an additional deterministic sensitivity analysis was conducted using the 95% confidence intervals (CIs) for upper and lower bound, where 95% CI were not available inputs were adjusted by ± 20%. Additionally, a two-way sensitivity analysis was conducted to assess the impact of correlated inputs on the model estimate for combined MB, by adjusting two key model inputs ± 20% simultaneously and testing for non-linear relationships between inputs.
Scenario analyses were conducted to explore the impact of the efficacy of the newly introduced antimicrobial. Five scenarios were considered, the first examined a scenario where all treatment lines have the same efficacy (90%), in the other analyses, the efficacy of the new antimicrobial was adjusted from 50 to 80% in 10% increments, whilst the efficacy of the current antimicrobials were unadjusted from the base case (piperacillin/tazobactam; 88% and meropenem; 87%).
3 Results
3.1 Outcomes of Continuing with No New Antimicrobial
Under current practice, the model projected that average AMR levels, modelled by pathogen and treatment, would increase by 7.81% after 10 years, from 23.46 to 31.27%, causing overall antimicrobial prophylaxis efficacy to decline by 4.95%. Over 10 years, it is estimated that, with the current treatment strategy, treating the HAIs of interest would involve 1,091,232 bed days at a hospitalization cost of $1.33 billion, and a loss of 180,989 LYs corresponding to 149,229 QALYs (Table 2). The estimated 10-year AMR projection in the prophylactic setting results in 19,679 additional infections, 1201 additional deaths, and 22,338 fewer QALYs based on the most common surgical procedures and immunosuppressing cancer chemotherapies in the USA (Table S11 of the ESM).
3.2 Value of Introducing a New Antimicrobial
The introduction of a new antimicrobial, in an AMS scenario where patients were diversified equally across all three treatment lines, would result in a proportional reduction of 9.03% from 31.27 to 28.44% in the projected resistance to the current antimicrobials considered in this analysis after 10 years (Table 2 and Fig. 2). In total, the health benefits of introducing a new antimicrobial, taking into consideration the impact on AMR and efficacy of antimicrobials in the treatment and prophylaxis setting, were estimated to be 11,444 QALYs gained in the first year versus current treatment strategies, increasing to 153,644 QALYs gained over 10 years (Table 2). The combined MB of $15.4 billion, at a WTP threshold of $100,000/QALY, represents the value to the US healthcare system in terms of QALYs gained and cost savings resulting from reduced healthcare resource utilization over 10 years (Table 2 and Fig. 3).
Projections of antimicrobial resistance (AMR) levels of current antimicrobials (piperacillin/tazobactam and meropenem) based on the current treatment strategy and an alternative treatment strategy introducing a new antimicrobial using a diversity approach; the proportional change in AMR of current antimicrobials between the current treatment and alternative treatment strategies
In the treatment setting, over 10 years, the alternative treatment strategy is estimated to save up to 49,608 bed days, translating to $64.3 million in hospitalisation costs, and resulting in a gain of 137,070 LYs (112,950 QALYs) in the treatment of HAIs (Table 2 and Fig. 4). The associated MB is estimated at $11.3 billion over 10 years.
In the prophylactic setting, 10 years after the introduction of a new antimicrobial, it is estimated that the reduction in AMR levels compared with existing treatment approaches would cause prophylaxis efficacy to increase by 1.82%. Over 10 years, this is estimated to result in 42,493 and 2594 cumulative infections and deaths avoided, respectively, during the most common surgical procedures and immunosuppressing cancer chemotherapies (Table 2 and Fig. 5A of the ESM). Based on infections and deaths avoided, 40,694 cumulative QALYs were estimated to be gained (Fig. 5B), equating to a MB of $4.1 billion over 10 years.
3.3 Sensitivity Analysis
A one-way sensitivity analysis showed that total MB was most sensitive to treatment efficacy estimates. For example, increased efficacy of the new antimicrobial resulted in more deaths avoided, leading to a large gain in QALYs and MB. Total MB ranged from $11.1 billion to $21.4 billion when the inputs for the three modelled treatments were varied by ±20% (Figs. S3 and S4 of the ESM). The model was less sensitive to adjusting estimates for baseline resistance and inputs for healthcare resource use during the treatment of infections; this is because costs associated with treating infections only account for a small proportion of overall MB, while a significant contribution is attributable to deaths avoided. Therefore, in scenarios where WTP per QALY gained was adjusted between $50,000 and $200,000, there was a significant change in MB, from $7.7 billion to $30.7 billion. A one-way sensitivity analysis assessing the impact of input uncertainty, where 95% CIs were used as upper and lower boundaries where available, the total MB ranged from $11.1 billion to $21.4 billion; this range was based on an assumed ±20% range for treatment efficacy, owing to 95% CIs not being reported and inputs being calculated as a weighted average. Because of the model’s sensitivity and uncertainty, inputs for treatment efficacy were explored further in exploratory scenarios.
A two-way sensitivity analysis showed a similar result in that treatment efficacy, life expectancy and utility (not infected) were the most sensitive inputs. The biggest impact on MB was seen when inputs for piperacillin/tazobactam and meropenem treatment efficacy were adjusted at the same time ($7.4–26.7 billion). The two-way sensitivity analysis demonstrated that the majority of inputs had an additive relationship (Fig. S6 of the ESM). Inputs for treatment efficacy, life expectancy and utility (not infected) showed a non-linear relationship (Fig. S6 of the ESM). Non-linear relationships can be expected in complex models such as dynamic transmission models; however, the scale of the variation shown was not considerable and is not a concern for compounding uncertainty in estimates for these inputs.
Scenario analyses were able to demonstrate that introducing a new antimicrobial with non-inferior efficacy still provides a MB of $13.8 billion. When the efficacy of the new antimicrobial was adjusted between 50 and 80% (with current treatment efficacy unadjusted from the base case [piperacillin/tazobactam; 88% and meropenem; 87%]), overall MB ranged from $5.6 billion to $13.0 billion, showing considerable benefits are still recognised even if the new antimicrobial is inferior to the current treatments (Table 12 of the ESM). Note, new treatments with efficacy below 50% are not likely to receive marketing authorisation; antimicrobials are commonly approved based on evidence from non-inferiority trials.
4 Discussion
The value of antimicrobial use within AMS strategies can be described by improvements in patient, population and health system outcomes. This study demonstrates a population-based mathematical model that incorporates STEDI value components to reveal the population-level value of antimicrobials. This de novo modelling framework recognises the clinical (reduced hospital length of stay, infections and deaths avoided) and economic benefits (reduced hospitalisation costs, monetary benefit associated with QALYs gained) associated with increased antimicrobial diversity within the treatment setting and the benefits that extend into the prophylactic setting in the USA. From a healthcare system perspective, these results support the value of investing in new antimicrobials and demonstrate that a considerable proportion of value can be recognised in the prophylactic setting. The STEDI value elements have only partly been implemented into economic evaluations; a recent analysis of ceftazidime with avibactam in the UK used the dynamic transmission model this current analysis was based upon and demonstrated how transmission and diversity elements can be evaluated; however, enablement value has only been discussed conceptually in the literature [26].
Over 10 years, the introduction of a new antimicrobial as part of an AMS strategy was estimated to result in a decrease in projected AMR levels from 31.27% (AMR under continuation with no new antimicrobial) to 28.44%. This reduction in AMR was estimated to be associated with an overall gain of 153,644 QALYs (treatment setting: 112,950; prophylactic setting: 40,694) and a MB of $15.4 billion (treatment setting: $11.3 billion; prophylactic setting: $4.1 billion) over 10 years. A deterministic sensitivity analysis highlighted the degree of uncertainty based on the model inputs; MB ranged from $11.1 billion to $21.4 billion. Model outcomes were most sensitive to estimates for treatment efficacy and resistance, emphasizing the importance of accurate clinical efficacy data and surveillance of AMR. However, exploratory scenarios showed that within this model framework, which considers population-level value elements, considerable value can still be realised even when the efficacy of the new antimicrobial is much lower than the currently available treatments ($5.6–13.0 billion at 50–80%).
Previous studies attempting to estimate the cost of AMR in the USA have predicted that AMR is responsible for treatment costs of between $2.2 billion and $4.4 billion annually [27,28,29]. In comparison, our estimates of $1.4 billion over 10 years (with current treatment options) appear conservative, as our analysis focuses on three indications and does not consider costs other than hospital length of stay. If our analysis was scaled up, by a factor of 5.6 from 19,396 HAIs to 109,491, to consider the treatment of all HAIs recorded in the National and State HAI progress report, regardless of indication or pathogen, the total MB of an effective antimicrobial over 10 years could be in the region of $87 billion.
Our analysis is not intended to consider further societal benefits and focuses on estimating the health economic benefit associated with new antimicrobials from the perspective of healthcare providers in the USA, considering treatment and prophylactic settings. However, the impacts of AMR are far reaching, entailing major societal cost drivers including a loss of productivity.
This analysis demonstrates the potential benefits of introducing a new antimicrobial increase over time, as projected resistance levels under the scenario with no new antimicrobial trend upwards. A 2018 Organization for Economic Co-operation and Development report estimated that the frequency of resistance could grow on average 23% between 2015 and 2030 [30]. This is considerably higher than the 10-year growth in AMR estimated from our model and suggests that the value of introducing a new antimicrobial is likely to be considerable in the long term. Our results, coupled with the Organization for Economic Co-operation and Development estimates, highlight the advantages of acting now, by incentivising the development of new antimicrobials, to provide patients and health systems with the benefits of lower AMR levels in the future.
Antimicrobial stewardship programs are organisational or healthcare system-wide approaches that aim to improve clinical outcomes and combat resistance by optimising how antimicrobials are used and prescribed [31]. In 2014, the Centers for Disease Control and Prevention called for implementation of an AMS program in all hospitals in the USA. A review of hospital AMS programs demonstrated that they have beneficial clinical and economic impacts driven by decreases in the length of hospital stay and antimicrobial expenditure [32]. However, AMS schemes alone may not be enough to counteract AMR [33]; encouraging investment in the development of new antimicrobials and their effective integration into clinical practice may also be needed.
As the benefits of new antimicrobials accrue to society in general and depend on restricting their use, the standard approach of manufacturers receiving a price for each unit sold is suboptimal. Several countries are exploring, or piloting, novel antimicrobial procurement and reimbursement models [34]. In the USA, the draft PASTEUR (Pioneering Antimicrobial Subscriptions to End Upsurging Resistance) Act proposes an antimicrobial subscription program. If enacted, this legislation will provide a substantial “pull incentive” for the development of new antimicrobials. Pull mechanisms aim to create market demand and/or revenue for products once they are approved and may be considered a reward for the results of R&D as opposed to the efforts themselves [35]. Pull incentives have been suggested as a key element in stimulating R&D for new antimicrobials [35] (Fig. 1). Their importance has been recognised in the success of the NHS England and NICE 2020 pilot program of health technology assessment and delinked payment model, in the UK, which is set to be expanded with maximum contract values proposed to increase from £10 million to £20 million [36]. Subscription style payments, linked to a broader value assessment, will be made to companies through an annual fixed fee that will not be linked to the volume of antimicrobial sold [36]. As a result, this program is likely not only to generate an R&D incentive, but also to encourage the use of antimicrobials in a manner consistent with good AMS practices, through de-linking price and volume. The success of such pull incentives depends upon effectively recognizing the holistic value of antimicrobials to the healthcare system. Wider adoption of incentives promoting the R&D of new antimicrobials could see substantial benefits to healthcare systems.
Whilst offering an innovative approach to quantifying antimicrobial value that broadens the methodological toolkit to include enablement when estimating STEDI value, the proposed model and analysis have some limitations that need to be considered when interpreting the analysis. To create a parsimonious tractable framework of AMR, the current de novo model was developed based on a previously developed and validated model where AMR dynamics were calibrated using UK data. The model-predicted outcomes for resistance were validated against historic data and showed the model is an acceptable predictor of resistance projections, but predictions slightly overestimated what was observed. Whilst the outcomes relating to resistance gain may be overestimated, the predicted incremental differences between scenarios are likely to be consistent. Furthermore, the approach to estimating value was conservative, as it was limited to the most common procedures and cancer chemotherapies used in the USA. It therefore, did not include all infections, pathogens, or surgical procedures, and did not consider the implications of freeing up hospital capacity (e.g. the response of planned admissions to a reduced need for intensive care beds as AMR levels are reduced); neither did it assess the benefits of reduced AMR in primary care. The sensitivity of the model to inputs and the impact on uncertainty was explored in a series of one-way sensitivity and scenario analyses. As the model is based on a dynamic model that utilises inputs derived from calibration, a probabilistic sensitivity analysis may produce spurious model outputs and therefore, was not conducted. It is important to recognise that the decision uncertainty of the presented analysis can only be considered within the context of the series of deterministic sensitivity analyses. Finally, the model captured the value of antimicrobials from the payer perspective only, without accounting for the impact of lost (or preserved) productivity, or the wider implications for the economy. Societal preferences of the general public in the UK highlight a high value is placed on approaches to reduce future AMR estimated to be £6–8 billion per year [37]. The coronavirus disease 2019 pandemic has clearly demonstrated the broad economic implications that an infectious disease may have [38,39,40], so it can be anticipated that benefits of novel antimicrobials are likely to reach far beyond the conservative estimates of the current model.
5 Conclusions
This research represents a step forward in the attempt to accurately estimate the population-level value of antimicrobials, accounting for transmission, diversity and enablement components of the STEDI framework. This quantifiable value should be reflected in policy investments to incentivise R&D and the timely access and appropriate use of new antimicrobials. However, further research is required to quantify the remaining STEDI value elements, spectrum and insurance, to support this effort there is also a requirement for increased surveillance and data collection.
References
Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55.
Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 22 Jan 2021.
HM Government. Tackling antimicrobial resistance 2019-2024. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf. Accessed 10 May 2021.
Infectious Diseases Society of America. PASTEUR Act will build antibiotic arsenal, protect existing medicines. 2020. https://www.idsociety.org/news-publications-new/articles/2020/pasteur-act-will-build-antibiotic-arsenal-protect-existing-medicines/. Accessed 16 Nov 2023.
Teillant A, Gandra S, Barter D, Morgan DJ, Laxminarayan R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect Dis. 2015;15(12):1429–37.
Jonas O, Irwin A, Berthe F, Le Gall F, Marquez P. Drug-resistant infections: a threat to our economic future (Vol. 2): final report (English). HNP/Agriculture Global Antimicrobial Resistance Initiative. Washington, DC: World Bank Group; 2017.
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12.
Projan SJ. Why is big Pharma getting out of antibacterial drug discovery? Curr Opin Microbiol. 2003;6(5):427–30.
Towse A, Sharma P. Incentives for R&D for new antimicrobial drugs. Int J Econ Business. 2011;18(2):331–50.
AMR Industry Alliance. AMR Industry Alliance, 2020 progress report. AMR Industry Alliance. Geneva; 2020.
Rothery C, Woods B, Schmitt L, Claxton K, Palmer S, Sculpher M. Framework for value assessment of new antimicrobials. Sheffield: EEPRU; 2018.
Colson AR, Morton A, Årdal C, Chalkidou K, Davies SC, Garrison LP, et al. Antimicrobial resistance: is health technology assessment part of the solution or part of the problem? Value Health. 2021;24(12):1828–34.
Outterson K, Rex JH. Evaluating for-profit public benefit corporations as an additional structure for antibiotic development and commercialization. Transl Res. 2020;220:182–90.
Gordon J, Darlington O, McEwan P, Lumley M, Taie A, Hicks M, et al. Estimating the value of new antimicrobials in the context of antimicrobial resistance: development and application of a dynamic disease transmission model. Pharmacoeconomics. 2020;38:857–69.
Jit M, Ng DHL, Luangasanatip N, Sandmann F, Atkins KE, Robotham JV, et al. Quantifying the economic cost of antibiotic resistance and the impact of related interventions: rapid methodological review, conceptual framework and recommendations for future studies. BMC Med. 2020;18(1):38.
Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–301.
Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol. 2020;41(1):1–18.
Centers for Disease Control and Prevention. NHSN reports. https://www.cdc.gov/nhsn/datastat/index.html. Accessed 19 Feb 2021.
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-111.
National Institute for Health and Care Excellence. Pyelonephritis (acute): antimicrobial prescribing [NG111]. 2018; https://www.nice.org.uk/guidance/ng111. Accessed 16 Nov 2023.
Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJC, Baron EJ, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–64.
Thomson Kenneth S, Moland ES. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2001;45(12):3548–54.
Sila Çetin Akhan FCOTHV. Conjugative resistance to tazobactam plus piperacillin among extended-spectrum beta-lactamase-producing nosocomial Klebsiella pneumoniae. Scand J Infect Dis. 2001;33(7):512–5.
Centers for Medicare & Medicaid Services. Cost reports 2019. https://www.cms.gov/research-statistics-data-and-systems/downloadable-public-use-files/cost-reports/. Accessed 7 Jan 2021.
Institute for Clinical Economic Review. ICER’s reference case for economic evaluations: principles and rationale. 2020. https://icer.org/wp-content/uploads/2020/10/ICER_Reference_Case_013120.pdf. Accessed 7 Jan 2021.
Gordon J, Gheorghe M, Goldenberg S, Miller R, Dennis J, Al-Taie A. Capturing Value Attributes in the economic evaluation of ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections in the United Kingdom. Pharmacoeconomics. 2023;41(12):1657–73.
Johnston KJ, Thorpe KE, Jacob JT, Murphy DJ. The incremental cost of infections associated with multidrug-resistant organisms in the inpatient hospital setting: a national estimate. Health Serv Res. 2019;54(4):782–92.
Michaelidis CI, Fine MJ, Lin CJ, Linder JA, Nowalk MP, Shields RK, et al. The hidden societal cost of antibiotic resistance per antibiotic prescribed in the United States: an exploratory analysis. BMC Infect Dis. 2016;16(1):655.
Thorpe KE, Joski P, Johnston KJ. Antibiotic-resistant infection treatment costs have Ddubled since 2002, now exceeding $2 billion annually. Health Aff (Millwood). 2018;37(4):662–9.
OECD. Stemming the superbug tide: just a few dollars more. Paris: OECD Health Policy Studies, OECD Publishing; 2018.
Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship programs. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html. Accessed 22 Jan 2021.
Nathwani D, Varghese D, Stephens J, Ansari W, Martin S, Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. 2019;8(1):35.
Aliabadi S, Anyanwu P, Beech E, Jauneikaite E, Wilson P, Hope R, et al. Effect of antibiotic stewardship interventions in primary care on antimicrobial resistance of Escherichia coli bacteraemia in England (2013–18): a quasi-experimental, ecological, data linkage study. Lancet Infect Dis. 2021;21(12):1689–700.
Gotham D, Moja L, van der Heijden M, Paulin S, Smith I, Beyer P. Reimbursement models to tackle market failures for antimicrobials: approaches taken in France, Germany, Sweden, the United Kingdom, and the United States. Health Policy. 2021;25(3):296–306.
Ferraro JS, Towse A, Mestre-Ferrandiz J. Office for Health Economics Briefing. Incentives for new drugs to tackle anti-microbial resistance. 2017. https://www.ohe.org/publications/incentives-new-drugs-tackle-anti-microbial-resistance. Accessed 19 Feb 2021.
National Institute for Health and Care Excellence. Models for the evaluation and purchase of antimicrobials. https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/models-for-the-evaluation-and-purchase-of-antimicrobials. Accessed 19 Feb 2021.
Dorgali MV, Longo A, Vass C, Shields G, Harrison R, Scarpa R, et al. A general public study on preferences and welfare impacts of antimicrobial resistance in the United Kingdom. Pharmaceconomics. 2022;40(1):65–76.
Chen J, Vullikanti A, Santos J, Venkatramanan S, Hoops S, Mortveit H, et al. Epidemiological and economic impact of COVID-19 in the US. Sci Rep. 2021;11(1):20451.
Maital S, Barzani E. The global economic impact of COVID-19: A summary of research. Samuel Neaman Institute for National Policy Research. 2020. https://www.neaman.org.il/EN/The-Global-Economic-Impact-of-COVID-19-A-Summary-of-Research.
Shang Y, Li H, Zhang R. Effects of pandemic outbreak on economies: evidence from business history context. Front Public Health. 2021;9:632043.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Pfizer Inc. who provided support for the model development/analysis and medical writing of this study.
Conflicts of Interest/Competing Interests
Amer Al-Taie and Maria Gheorghe are employees of Pfizer Inc. and hold stocks and stock options from Pfizer Inc. Jason Gordon, Cale Harrison, James Dennis and Ryan Miller are employees of Health Economics and Outcomes Research Ltd. Health Economics and Outcomes Research Ltd. received fees from Pfizer Inc. in relation to this study. Simon Goldenberg has received consulting fees from Enterobiotix, Shinogi and Tillotts Pharma and speaker’s fees from Tillotts Pharma. Lotte Steuten is an employee of the Office of Health Economics. The Office of Health Economics has received payments for contract research from the Wellcome Trust, Pfizer, Shionogi, GSK and the Association of the British Pharmaceutical Industry, for work related to antimicrobial resistance. Payments for conference presentations were also received by the Office of Health Economics for work related to antimicrobial resistance. Sumanth Gandra has no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
JG and AT conceptualised and designed the study. RM was responsible for the data analysis. All authors contributed to the interpretation of results, preparation and review of the manuscript, and approval of the final manuscript for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gordon, J., Gheorghe, M., Harrison, C. et al. Estimating the Treatment and Prophylactic Economic Value of New Antimicrobials in Managing Antibiotic Resistance and Serious Infections for Common Pathogens in the USA: A Population Modelling Study. PharmacoEconomics 42, 329–341 (2024). https://doi.org/10.1007/s40273-023-01337-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01337-9