Abstract
Physiologically based pharmacokinetic (PBPK) modeling can be an attractive tool to increase the evidence base of pediatric drug dosing recommendations by making optimal use of existing pharmacokinetic (PK) data. A pragmatic approach of combining available compound models with a virtual pediatric physiology model can be a rational solution to predict PK and hence support dosing guidelines for children in real-life clinical care, when it can also be employed by individuals with little experience in PBPK modeling. This comes within reach as user-friendly PBPK modeling platforms exist and, for many drugs and populations, models are ready for use. We have identified a list of drugs that can serve as a starting point for pragmatic PBPK modeling to address current clinical dosing needs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
More than half of all drugs are prescribed off-label to children. Physiologically based pharmacokinetic (PBPK) modeling is a valuable approach to support dosing in this special population. |
The increasing availability of compound and physiology models offers the opportunity to expedite model-informed dosing following a pragmatic approach as high-level expertise becomes less essential. |
To ultimately develop model-informed dosing for clinical care, we present a list of drugs for which PBPK modeling could support pediatric dosing guidelines. |
1 Introduction
The establishment of legislative and regulatory frameworks has resulted in an expansion of pediatric clinical trials since the early 2000s, but still approximately 50% of all drugs prescribed to children are used off-label [1, 2]. Hence, clinicians regularly consult their national pediatric formularies or commercial sources, such as the Harriet Lane Handbook, Micromedex, and Lexicomp, for guidance. In the Netherlands, the Dutch Pediatric Formulary (DPF) was launched in 2008 and is consulted by > 10,000 unique visitors on a daily basis [3]. Knowledge-based dosing guidelines of the DPF are established based on a careful screening of scientific literature. An analysis of the level of evidence showed that one-third of all dosing recommendations in the DPF is backed by only a low level of evidence (i.e., based on comparative research, non-comparative research or expert consensus) [4].
Pediatric dosing recommendations can be supported with pediatric pharmacokinetic (PK) data [5]. The importance of pediatric PK studies is widely recognized. Initiatives such as the Pediatric Trials Network (PTN) and the Priority List of Needs in Pediatric Therapeutics (Best Pharmaceuticals for Children Act, BPCA), as well as the Inventory of Pediatric Therapeutic Needs (European Medicines Agency, EMA) show that regulatory authorities encourage pediatric research. These initiatives highlight which drugs and indications require further research in children and aim for label change [6, 7]. Even when pediatric PK data become available, development of a PK-based dosing recommendation requires comprehensive interpretation of the data obtained from study subjects who often under-represent broader pediatric populations. PK studies often include a small number of pediatric subjects or do not cover the entire pediatric age range [8]. Inadequate interpretation and the necessity to subsequently validate PK-based dosing recommendations often leaves these data underused and hence impedes efficient clinical implementation. One approach that can be employed to unleash the value of existing PK data to support dosing recommendations for all pediatric age groups is physiologically based pharmacokinetic (PBPK) modeling. In silico PBPK modeling has gained momentum over the past decades, especially in the process of drug development [9, 10], and is widely recognized as a valuable tool to extrapolate drug PK throughout different stages of childhood and hence further guide dosing [11]. In combination with already available, but often small, clinical pediatric PK datasets, PBPK modeling allows for the generation of a larger evidence base for drug dosing in pediatrics. This evidence may either result in a label change or may serve as supportive evidence for off-label drug use.
2 Physiologically Based Pharmacokinetic (PBPK) Modeling
A PBPK model comprises several compartments representing human tissues and the systemic circulation and is built based on anatomical, biochemical, and physiological information. Intercompartmental drug exchange is described by mathematical equations. An advanced understanding of human physiology and of key pathological processes affecting PK processes has enabled the creation of a diverse set of virtual physiology models representing specific populations. These physiology models can be updated constantly through incorporation of new physiological information as this comes available. A pediatric model, for example, has been developed in several user-friendly PBPK modeling platforms with past and emerging knowledge on developmental physiology and ontogeny of drug transporters and metabolizing enzymes [12,13,14]. Even more sophisticated is the recent development of a physiology model representing preterm neonates, built using age-dependent maturation and ontogeny patterns reported in literature [15,16,17]. In a set of virtual subjects (‘physiology model’), drug-specific physicochemical, binding, permeation, active transport, and metabolism properties (captured in a ‘compound model’) dictate the absorption, distribution, metabolism, and excretion of a specific drug. The user can then design a virtual clinical trial by adjusting subject demographics (e.g., age range and gender) and simulate drug PK under different dosing strategies. Next, based on simulations of PK, dosing regimens can be established when target concentrations are known or when it is decided to base these on adult exposure matching.
3 Pre- and Post-Market Utilization of Pediatric PBPK Modeling
PBPK modeling is increasingly applied in pre-market safety and efficacy studies to guide first-in-pediatric dose selection, its use being driven by the introduction of the Pediatric Study Plan (PSP) in the USA and the Paediatric Investigation Plan (PIP) in the European Union in the 2000s [18, 19]. Both the US Food and Drug Administration (FDA) and the EMA support the use of PBPK models in regulatory submissions in conjunction with clinical studies [20, 21]. As such, PBPK modeling has taken a prominent place in regulatory applications in the process of drug development, supporting optimal use of new drugs in pediatric patients. Yet, many healthcare professionals deal with off-label prescribing of relatively old drugs. These drugs are less likely to be studied through clinical trials, for all indications or all age groups, due to financial or organizational hurdles, a lack of resources or an insufficient number of pediatric patients. PBPK modeling is also frequently employed in an academic research setting, for instance to gain insights into PK processes like renal transporter ontogeny or developmental changes in biliary drug excretion [22, 23]. However, there is much less attention for the use of PBPK modeling to generate dosing advices for clinical practice. In our opinion, PBPK modeling is an attractive tool for establishing pediatric drug dosing recommendations in the post-marketing phase, as well. For this we have four main reasons (Fig. 1):
-
1.
The increasing availability of compound and physiology models in software platforms, repositories, and scientific literature enables a pragmatic and relatively easy PBPK modeling approach. Software platforms become more and more user friendly and intuitive, and it is envisaged that high-level expertise becomes less essential for understanding the content and utility of PBPK modeling and running PBPK model simulations. Section 4 explains this concept in more detail.
-
2.
Conducting a pediatric clinical trial takes months or years and the costs to prospectively study one drug for one indication in a formal pediatric development plan may amount to 20 million USD [24]. In contrast, PBPK modeling is highly mechanistic and can be used to extrapolate PK and guide dosing in pediatric subjects of different age groups in an efficient and cost-effective manner. Especially in situations of a high unmet medical need, it is of eminent importance to save time. A case example is presented in Sect. 5.
-
3.
Many drugs are used off-label in pediatrics. Even though pediatric regulations now mandate pediatric clinical trials for new drugs, for many old drugs this situation will likely not change significantly, despite efforts such as the BPCA. For these drugs, PBPK model simulations can be used as quality evidence to support or establish off-label dosing recommendations.
-
4.
A scenario in which altered PK is expected can necessitate a dose adjustment. With PBPK modeling, it is not only possible to study the effect of age on drug PK, an additional effect of, for example, co-medication, ethnicity, renal impairment or obesity on a drug’s PK profile can also be investigated [25,26,27].
4 A Pragmatic PBPK Modeling Approach
Pediatric PBPK modeling matures rapidly. In 2020, the number of scientific publications on pediatric PBPK modeling was 34 compared to one publication in 2005 [28]. Yet, many more physiology and compound models are available in repositories. The availability of these models offers the opportunity to expedite model-informed dosing following a pragmatic approach as specific expertise on how to code and parameterize such models were left for modeling experts. Also, using existing models is efficient since developing compound and physiology models from scratch and thorough quality assessment can be time-consuming [29]. It should be noted that when following this approach, quality of both compound and physiology models is assumed to be yet assessed and considered adequate. A detailed description of steps involved in quality assurance of PBPK modeling, from platform validation to model application, has recently been published by Frechen and Rostami-Hodjegan [30].
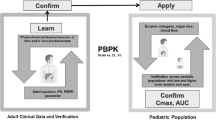
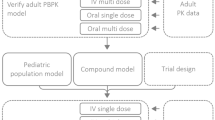
In principle, the distinct separation of drug-specific data and physiological data in a PBPK modeling framework should allow the user to simulate PK of a specific drug in any defined virtual population by coupling a compound model, without any change, to any physiology model of interest (e.g., pediatrics). Such a pragmatic approach is a rational solution to support dosing guidelines for children in real-life clinical care. A widely supported structured workflow of using adult PBPK models for the prediction of pediatric PK and, hence, pediatric dosing is visualized in Fig. 2 [31,32,33,34].
A pragmatic approach for pediatric physiologically based pharmacokinetic (PBPK) modeling. Drug-specific physicochemical properties are defined in the compound model and age-dependent anatomical and physiological parameters are defined in the virtual physiology models. The trial design includes, amongst others, a description of the dosing schedule and the age and gender of the virtual subjects and can be adjusted to reflect the clinical study design of interest (indicated with the dashed border)
5 Balancing Model Credibility with Medical Need
A pragmatic PBPK modeling approach can only be employed when there is sufficient confidence in the quality of the models used. Pediatric models are often developed in trustworthy PBPK platforms, but research on developmental physiology and pharmacology is a continuous process and the data incorporated in the models, such as enzyme ontogeny patterns, may therefore need regular updates [35]. With respect to compound models, the level of confidence in their quality depends on how detailed and accurate the physicochemical and PK properties of a drug are defined in the model and steps taken to validate the model. Evaluation of physiology and compound models is critical to ensure effective applications (Fig. 2).
In any case, adequate incorporation of drug elimination pathways is essential when using a compound model for simulations of PK in adults first and subsequently in pediatric subjects (i.e., the pragmatic PBPK modeling approach as presented in Sect. 4). Enzyme ontogeny affects PK, so clearance needs to be described on the enzyme level—rather than total body clearance—together with enzyme-specific ontogeny profiles. Ideally, parameters describing enzyme-specific Michaelis–Menten kinetics should be incorporated in the model to be able to capture possible enzyme saturation. Also, the relative contribution of different eliminating pathways may be age-dependent and should therefore ideally be confirmed with data from age-specific mass balance studies [32, 33].
In general, PBPK model credibility can be examined via verification and validation activities, and the rigor of these activities is dependent on the risks a model-informed decision entails (i.e., ‘model risk’). Model risk is determined by both the ‘model influence’ and the ‘decision consequence’ [36]. In case clinical PK data are absent for all pediatric age groups, a PBPK model simulation in itself provides substantial evidence for a certain dose (given the totality of available evidence), and the model influence is considered high. If there is also a high chance that inaccurate PBPK model simulations have severe consequences, for instance toxicity in preterm neonates, the decision consequence is thought to be high too. Taken together, in that case the high model risk requires rigorous verification and validation activities to demonstrate model credibility. On the other hand, model risk can be considered low in cases where there is uncertainty in PBPK model predictions of exposure but the drug has a wide therapeutic window. Careful balancing of model credibility with the medical need for a dosing recommendation is essential.
We showed the feasibility of the pragmatic PBPK modeling approach during the early phase of the COVID-19 pandemic, when we developed dosing guidelines using a recently published chloroquine compound model and a pediatric model. In this case, only PK data from malaria-infected children were available for model verification and the complete lack of PK data for children < 6 months of age precluded the assessment of model performance for prediction of PK in this age group. However, the Simcyp® pediatric model has extensively been verified for other drugs, also cleared via cytochrome P450 3A, cytochrome P450 2C8, and the kidneys, in this age range. In order to get dosing recommendations for this critical case, we had to accept a certain level of uncertainty [37]. Dosing recommendations were established based on adult exposure matching. Because of the inability to assess real-life efficacy and safety with PBPK modeling, clinical experience with the model-informed dose is essential to uncover potential specific exposure-related efficacy and safety issues that were not expected based on adult data.
6 Moving Forward
Widespread implementation of the pragmatic pediatric PBPK modeling approach is still lacking, which is also related to the fact that the feasibility of this approach is not yet fully elucidated. In order to move forward, a standard protocol on how to employ this approach is essential, including a checklist for thorough assessment of model quality, a clear description of the modeling steps required, and guidance on how to interpret and report results of PBPK model simulations. Also, a decision framework for clinical implementation of a model-informed dose is warranted, as carefully balancing the risks with the benefits is not straight-forward, involves many stakeholders, and is a drug-, indication-, and patient-specific effort. Such a framework facilitates integration of modeling information into the workflow of the Pediatric Formularies in a structured manner. Regulatory agencies have dedicated PBPK teams to examine model simulations submitted with new drug applications, but who takes the responsibility and liability for model-informed off-label drug dosing recommendations? A review board with experts on PBPK modeling and communication of the strengths, limitations, and applications among non-PBPK modeling experts through introductory trainings are key to moving forward and to obtaining scientifically sound dosing advices implemented in clinical care.
To ultimately develop model-informed dosing for clinical care, we here present a list of drugs for which PBPK modeling could support pediatric dosing guidelines. This list serves as an example of a starting point. We selected these pharmaceuticals as follows: As a first step, the Model List of Essential Medicines for Children of the World Health Organization (WHO) was screened [20]. We prioritized our selection to only include drugs that are on this list. Vaccines and dietary supplements were excluded. Next, drugs were selected if they were listed in the European Pediatric Formularies (The Netherlands: www.kinderformularium.nl; Germany: www.kinderformularium.de; Austria: www.kindermedika.at; Norway: www.koble.info). These formularies provide pediatric dosing guidelines for > 800 drugs, representing a large proportion of all drugs used in children globally [38]. The final list with drugs relevant for pediatric PBPK modeling can be found in the Online Supplementary Information (Resource 1).
Next, critical appraisal of the drugs on the list can be useful to further pinpoint the need for and feasibility of conducting PBPK modeling. Different approaches can be followed at this point:
-
A.
Further prioritization of the drugs can be based on information provided by BPCA’s Priority List of Needs in Pediatric Therapeutics [6]. The utility of this list is apparent as it reports information on ongoing PK studies and drug label changes. The availability of pediatric PK data can be viewed as a prerequisite at this point, as it can be deployed to ensure adequate model performance. Relevant information of the BPCA is listed in the OSM (Resource 1). Selected drugs were categorized highlighting the current potential value of pediatric PBPK modeling (i.e., high, moderate, or low).
-
B.
As stated before, the level of evidence for efficacy has been assessed for all drug dosing recommendations in the European Pediatric Formularies. This information, together with information from a literature search on available pediatric PK data, can be used to identify which dosing recommendations can be supported by PBPK modeling [4].
-
C.
Quality of the compound model is a relevant factor to take into consideration when employing the pragmatic approach (see Sect. 4). Predefined and thoroughly validated compound models are available in dedicated PBPK modeling software platforms and it can be decided to prioritize drugs for which such a compound model is available.
7 Conclusion
Our proposed pragmatic PBPK modeling approach presents a promising, encouraging and time-saving strategy to predict PK and guide dosing in pediatric clinical care. Despite increased complexity of the PBPK models, PBPK modeling platforms are becoming more user friendly. High-level PBPK modeling expertise is less essential at the clinical implementation level, when validated models are available together with standardized protocols on how to conduct PBPK modeling and interpret and report the results.
Despite the fact that clinical assessment of model-informed dosing recommendations is advised, it is evident that pediatric clinical care can significantly benefit from the rapid progress in the field of PBPK modeling. Implementing PBPK model-informed pediatric dosing enables efficient and evidence-based decision-making and is within reach, although a joint effort with multiple research teams and an internationally accessible platform is needed to share information on pediatric PBPK modeling and eventually implement model-informed doses in clinical practice. We envision that digital pieces of evidence will slowly but surely contribute to effective and safe pharmacotherapy in pediatrics.
References
Turner MA, Catapano M, Hirschfeld S, Giaquinto C, Global Research in P. Paediatric drug development: the impact of evolving regulations. Adv Drug Deliv Rev. 2014;73:2–13.
Wang X, Zhang ZY, Arora S, Wang J, Lu S, Powers D, et al. Effects of Rolapitant Administered Intravenously on the Pharmacokinetics of a Modified Cooperstown Cocktail (Midazolam, Omeprazole, Warfarin, Caffeine, and Dextromethorphan) in Healthy Subjects. J Clin Pharmacol. 2018;58(8):1074–83.
van der Zanden TM, de Wildt SN, Liem Y, Offringa M, de Hoog M, Dutch Paediatric Pharmacotherapy Expertise Network N. Developing a paediatric drug formulary for the Netherlands. Arch Dis Child. 2017;102(4):357–61.
van der Zanden TM, Smeets NJL, de Hoop-Sommen M, Schwerzel MFT, Huang HJ, Barten LJC et al. Off-label, but on-evidence? A review of the level of evidence for pediatric pharmacotherapy. Clin Pharmacol Ther. 2022. https://doi.org/10.1002/cpt.2736
Dunne J, Rodriguez WJ, Murphy MD, Beasley BN, Burckart GJ, Filie JD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011;128(5):e1242–9.
National Institutes of Health (NIH). Best Pharmaceuticals for Children Act (BPCA) Priority List of Needs in Pediatric Therapeutics 2020-2021. https://www.nichd.nih.gov/research/supported/bpca/prioritizing-pediatric-therapies. Accessed Jan 2022.
European Medicines Agency (EMA). Needs for Paediatric Medicines. https://www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/needs-paediatric-medicines. Accessed Jan 2022.
Barker CIS, Standing JF, Kelly LE, Hanly Faught L, Needham AC, Rieder MJ, et al. Pharmacokinetic studies in children: recommendations for practice and research. Arch Dis Child. 2018;103(7):695–702.
Zhang X, Yang Y, Grimstein M, Fan J, Grillo JA, Huang SM, et al. Application of PBPK modeling and simulation for regulatory decision making and its impact on US prescribing information: an update on the 2018–2019 Submissions to the US FDA’s Office of Clinical Pharmacology. J Clin Pharmacol. 2020;60(Suppl 1):S160–78.
Luzon E, Blake K, Cole S, Nordmark A, Versantvoort C, Berglund EG. Physiologically based pharmacokinetic modeling in regulatory decision-making at the European Medicines Agency. Clin Pharmacol Ther. 2017;102(1):98–105.
Perry C, Davis G, Conner TM, Zhang T. Utilization of physiologically based pharmacokinetic modeling in clinical pharmacology and therapeutics: an overview. Curr Pharmacol Rep. 2020;6(3):71–84.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45(9):931–56.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45(10):1013–34.
Bjorkman S. Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol. 2005;59(6):691–704.
Abduljalil K, Pan X, Pansari A, Jamei M, Johnson TN. Preterm physiologically based pharmacokinetic model. Part II: applications of the model to predict drug pharmacokinetics in the preterm population. Clin Pharmacokinet. 2020;59(4):501–18.
Abduljalil K, Pan X, Pansari A, Jamei M, Johnson TN. A preterm physiologically based pharmacokinetic model. Part I: Physiological parameters and model building. Clin Pharmacokinet. 2020;59(4):485–500.
Claassen K, Thelen K, Coboeken K, Gaub T, Lippert J, Allegaert K, et al. Development of a physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr Pharm Des. 2015;21(39):5688–98.
Wang K, Jiang K, Wei X, Li Y, Wang T, Song Y. Physiologically based pharmacokinetic models are effective support for pediatric drug development. AAPS PharmSciTech. 2021;22(6):208.
Mehrotra N, Bhattaram A, Earp JC, Florian J, Krudys K, Lee JE, et al. Role of quantitative clinical pharmacology in pediatric approval and labeling. Drug Metab Dispos. 2016;44(7):924–33.
Lander CM, Donnan GA, Bladin PF, Vajda FJ. Some aspects of the clinical use of clonazepam in refractory epilepsy. Clin Exp Neurol. 1979;16:325–32.
Bottaï T, Hüe B, Hillaire-Buys D, Barbe A, Alric R, Pouget R, et al. Clonazepam in acute mania: time-blind evaluation of clinical response and concentrations in plasma. J Affect Disord. 1995;36(1–2):21–7.
Cristea S, Krekels EHJ, Rostami-Hodjegan A, Allegaert K, Knibbe CAJ. The influence of drug properties and ontogeny of transporters on pediatric renal clearance through glomerular filtration and active secretion: a simulation-based study. Aaps J. 2020;22(4):87.
Johnson TN, Jamei M, Rowland-Yeo K. How does in vivo biliary elimination of drugs change with age? Evidence from in vitro and clinical data using a systems pharmacology approach. Drug Metab Dispos. 2016;44(7):1090–8.
European Commission. State of paediatric medicines in the EU—10 years of the EU paediatric regulation. COM (2017) 626. https://www.ema.europa.eu/en/human-regulatory/overview/paediatric-medicines/paediatric-regulation. Accessed Jan 2022.
Ford JL, Gerhart JG, Edginton AN, Yanovski JA, Hon YY, Gonzalez D. Physiologically based pharmacokinetic modeling of metformin in children and adolescents with obesity. J Clin Pharmacol. 2022;62(8):960–69.
Yao XT, Liu XL, Tu SQ, Li XB, Lei ZH, Hou Z, et al. Development of virtual Chinese pediatric population physiological model targeting specific metabolism and kidney elimination pathways. Front Pharmacol. 2021;12:648697.
Xu JW, Lin RF, Chen Y, You X, Huang PF, Lin CH. Physiologically based pharmacokinetic modeling and dose adjustment of teicoplanin in pediatric patients with renal impairment. J Clin Pharmacol. 2022;62(5):620–30.
Johnson TN, Small BG, Rowland YK. Increasing application of pediatric physiologically based pharmacokinetic models across academic and industry organizations. CPT Pharmacomet Syst Pharmacol. 2022;11(3):373–83.
Ezuruike U, Zhang M, Pansari A, De Sousa Mendes M, Pan X, Neuhoff S, et al. Guide to development of compound files for PBPK modeling in the Simcyp population-based simulator. CPT Pharmacomet Syst Pharmacol. 2022;11(7):805–21.
Frechen S, Rostami-Hodjegan A. Quality assurance of PBPK modeling platforms and guidance on building, evaluating, verifying and applying PBPK models prudently under the umbrella of qualification: why, when, what, how and by whom? Pharm Res. 2022;39(8):1733–48.
Yellepeddi V, Rower J, Liu X, Kumar S, Rashid J, Sherwin CMT. State-of-the-art review on physiologically based pharmacokinetic modeling in pediatric drug development. Clin Pharmacokinet. 2019;58(1):1–13.
Verscheijden LFM, Koenderink JB, Johnson TN, de Wildt SN, Russel FGM. Physiologically-based pharmacokinetic models for children: Starting to reach maturation? Pharmacol Ther. 2020;211: 107541.
Johnson TN, Small BG, Berglund EG, Rowland YK. A best practice framework for applying physiologically-based pharmacokinetic modeling to pediatric drug development. CPT Pharmacomet Syst Pharmacol. 2021;10(9):967–72.
Lin WY, Yan J-H, Heimbach T, He H. Pediatric physiologically based pharmacokinetic model development: current status and challenges. Curr Pharmacol Rep. 2018;4:10.
van Hoogdalem MW, Johnson TN, McPhail BT, Kamatkar S, Wexelblatt SL, Ward LP, et al. Physiologically-based pharmacokinetic modeling to investigate the effect of maturation on buprenorphine pharmacokinetics in newborns with neonatal opioid withdrawal syndrome. Clin Pharmacol Ther. 2022;111(2):496–508.
Kuemmel C, Yang Y, Zhang X, Florian J, Zhu H, Tegenge M, et al. Consideration of a credibility assessment framework in model-informed drug development: potential application to physiologically-based pharmacokinetic modeling and simulation. CPT Pharmacomet Syst Pharmacol. 2020;9(1):21–8.
Verscheijden LFM, van der Zanden TM, van Bussel LPM, de Hoop-Sommen M, Russel FGM, Johnson TN, et al. Chloroquine dosing recommendations for pediatric COVID-19 supported by modeling and simulation. Clin Pharmacol Ther. 2020;108(2):248–52.
van der Zanden TM, Neubert A, Zahn J, Wimmer S, de Hoop-Sommen M, Rosness T, et al. ESDPPP 2019 Poster. P101 Extending the Dutch Paediatric Formulary across Europe: successful development of country specific, parallel, paediatric drug formularies.
Acknowledgements
We thank Dr. Ping Zhao and Tjitske van der Zanden for useful discussions that improved the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This publication is based on research funded by the Bill & Melinda Gates Foundation (Grant number: INV-001822). The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Ethics approval
No ethical approval is required.
Consent to participate
Not applicable (no subjects were involved).
Consent for publication
Not applicable (no subjects were involved).
Statement of data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Author contributions
All authors contributed to outline of the Current Opinion. The first draft of the manuscript was written by Jolien J.M. Freriksen and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Freriksen, J.J.M., van der Heijden, J.E.M., de Hoop-Sommen, M.A. et al. Physiologically Based Pharmacokinetic (PBPK) Model-Informed Dosing Guidelines for Pediatric Clinical Care: A Pragmatic Approach for a Special Population. Pediatr Drugs 25, 5–11 (2023). https://doi.org/10.1007/s40272-022-00535-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00535-w