Abstract
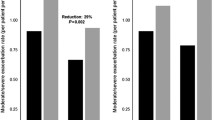
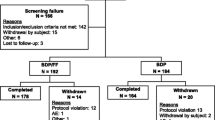
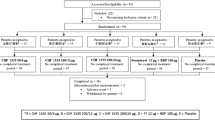
Extrafine beclometasone dipropionate/formoterol 100/6 and 200/6 μg fixed-dose combination (FDC) is available in the EU as a pressurized metered-dose inhaler (Fostair®, Foster®, Formodual®, Innovair®, Inuvair®, Inuxair®, Combair®) and a dry-powder inhaler (Fostair NEXThaler®, Foster NEXThaler®, Formodual NEXThaler®, Innovair NEXThaler®, Inuvair NEXThaler®). It is an effective, convenient and well-tolerated option for maintenance, or maintenance and reliever therapy in patients with asthma, as well as for the symptomatic treatment of patients with severe chronic obstructive pulmonary disease (COPD). Extrafine beclometasone/formoterol is generally well tolerated, improves lung function and asthma control and reduces COPD exacerbations in these patients in clinical studies. It is generally more effective than beclometasone and formoterol monotherapies in clinical studies and generally provided better asthma control, reduced the use of rescue medication and improved health-related quality of life compared with large-particle FDCs in real-world studies.
Similar content being viewed by others
References
Miller-Larsson A, Selroos O. Advances in asthma and COPD treatment: combination therapy with inhaled corticosteroids and long-acting beta 2-agonists. Curr Pharm Des. 2006;12(25):3261–79.
Global strategy for asthma management and prevention: updated 2017. Bethesda: Global Initiative for Asthma (GINA); 2017.
Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2017. Bethesda: Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2017.
British guideline on the management of asthma. London: British Thoracic Society; 2016.
Barnes PJ. A single inhaler for asthma? Am J Respir Crit Care Med. 2005;171(2):95–6.
Nicolini G, Scichilone N, Bizzi A, et al. Beclomethasone/formoterol fixed combination for the management of asthma: patient considerations. Ther Clin Risk Manag. 2008;4(5):855–64.
Fostair (beclometasone dipropionate/formoterol) 100/6 inhalation solution: summary of product characteristics. Manchester: Chiesi Limited; 2016.
Fostair (beclometasone dipropionate/formoterol) 200/6 inhalation solution: summary of product characteristics. Manchester: Chiesi Limited; 2017.
Fostair NEXThaler (beclometasone dipropionate/formoterol) 100/6 inhalation solution: summary of product characteristics. Manchester: Chiese Limited; 2016.
Fostair NEXThaler (beclometasone dipropionate/formoterol) 200/6 inhalation solution: summary of product characteristics. Manchester: Chiesi Limited; 2016.
Bousquet J, Poli G, Acerbi D, et al. Systemic exposure and implications for lung deposition with an extra-fine hydrofluoroalkane beclometasone dipropionate/formoterol fixed combination. Clin Pharmacokinet. 2009;48(6):347–58.
Fabbri LM, Nicolini G, Olivieri D, et al. Inhaled beclometasone dipropionate/formoterol extra-fine fixed combination in the treatment of asthma: evidence and future perspectives. Expert Opin Pharmacother. 2008;9(3):479–90.
Dhillon S, Keating GM. Beclometasone dipropionate/formoterol: in an HFA-propelled pressurised metered-dose inhaler. Drugs. 2006;66(11):1475–83 (discussion 84–85).
De Backer W, Devolder A, Poli G, et al. Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients. J Aerosol Med Pulm Drug Deliv. 2010;23(3):137–48.
Corradi M, Chrystyn H, Cosio BG, et al. NEXThaler, an innovative dry powder inhaler delivering an extrafine fixed combination of beclometasone and formoterol to treat large and small airways in asthma. Expert Opin Drug Deliv. 2014;11(9):1497–506.
Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur Respir J. 2002;19(1):182–91.
O’Connor BJ, Collarini S, Poli G, et al. Rapid effects of extrafine beclomethasone dipropionate/formoterol fixed combination inhaler on airway inflammation and bronchoconstriction in asthma: a randomised controlled trial. BMC Pulm Med. 2011;11:60.
Singh D, Corradi M, Bindi E, et al. Relief of methacholine-induced bronchospasm with extrafine beclomethasone dipropionate/formoterol in comparison with salbutamol in asthma. Pulm Pharmacol Ther. 2012;25(5):392–8.
Singh D, Nicolini G, Bindi E, et al. Extrafine beclomethasone/formoterol compared to fluticasone/salmeterol combination therapy in COPD. BMC Pulm Med. 2014;14:43.
Papi A, Paggiaro P, Nicolini G, et al. Beclomethasone/formoterol vs fluticasone/salmeterol inhaled combination in moderate to severe asthma. Allergy. 2007;62(10):1182–8.
De Backer J, Vos W, Vinchurkar S, et al. The effects of extrafine beclometasone/formoterol (BDP/F) on lung function, dyspnea, hyperinflation, and airway geometry in COPD patients: novel insight using functional respiratory imaging. J Aerosol Med Pulm Drug Deliv. 2015;28(2):88–99.
Tzani P, Crisafulli E, Nicolini G, et al. Effects of beclomethasone/formoterol fixed combination on lung hyperinflation and dyspnea in COPD patients. Int J Chron Obstruct Pulmon Dis. 2011;6:503–9.
Calverley PM, Kuna P, Monso E, et al. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respir Med. 2010;104(12):1858–68.
Wedzicha JA, Singh D, Vestbo J, et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108(8):1153–62.
Lewis DA, Brambilla G, Church T, et al. BDP and formoterol association within a combination HFA solution MDI. Respir Drug Deliv. 2006;3:939–42.
Corradi M, Spinola M, Petruzzelli S, et al. High-dose beclometasone dipropionate/formoterol fumarate in fixed-dose combination for the treatment of asthma. Ther Adv Respir Dis. 2016;10(5):492–502.
Huchon G, Magnussen H, Chuchalin A, et al. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103(1):41–9.
Papi A, Paggiaro PL, Nicolini G, et al. Beclomethasone/formoterol versus budesonide/formoterol combination therapy in asthma. Eur Respir J. 2007;29(4):682–9.
Paggiaro P, Corradi M, Latorre M, et al. High strength extrafine pMDI beclometasone/formoterol (200/6 μg) is effective in asthma patients not adequately controlled on medium-high dose of inhaled corticosteroids. BMC Pulm Med. 2016;16(1):180.
Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med. 2013;1(1):23–31.
Barnes N, van Noord JA, Brindicci C, et al. Stepping-across controlled asthmatic patients to extrafine beclometasone/formoterol combination. Pulm Pharmacol Ther. 2013;26(5):555–61.
Papi A, Nicolini G, Crimi N, et al. Step-down from high dose fixed combination therapy in asthma patients: a randomized controlled trial. Respir Res. 2012;13:54.
Terzano C, Cremonesi G, Girbino G, et al. 1-year prospective real life monitoring of asthma control and quality of life in Italy. Respir Res. 2012;13:112.
Allegra L, Cremonesi G, Girbino G, et al. Real-life prospective study on asthma control in Italy: cross-sectional phase results. Respir Med. 2012;106(2):205–14.
Kuna P, Kuprys-Lipinska I, Debowski T. Control of asthma in adults treated with beclomethasone and formoterol in extrafine particle formulation in a real-life setting in Poland: the CASPER noninterventional, observational trial. Pol Arch Med Wewn. 2015;125(10):731–40.
Marth K, Spinola M, Kisiel J, et al. Treatment response according to small airway phenotypes: a real-life observational study. Ther Adv Respir Dis. 2016;10(3):200–10.
Brusselle G, Peche R, Van den Brande P, et al. Real-life effectiveness of extrafine beclometasone dipropionate/formoterol in adults with persistent asthma according to smoking status. Respir Med. 2012;106(6):811–9.
Popov TA, Petrova D, Kralimarkova TZ, et al. Real life clinical study design supporting the effectiveness of extra-fine inhaled beclomethasone/formoterol at the level of small airways of asthmatics. Pulm Pharmacol Ther. 2013;26(6):624–9.
Muller V, Galffy G, Eszes N, et al. Asthma control in patients receiving inhaled corticosteroid and long-acting β2-agonist fixed combinations. A real-life study comparing dry powder inhalers and a pressurized metered dose inhaler extrafine formulation. BMC Pulm Med. 2011;11:40.
Price D, Small I, Haughney J, et al. Clinical and cost effectiveness of switching asthma patients from fluticasone-salmeterol to extra-fine particle beclometasone-formoterol: a retrospective matched observational study of real-world patients. Prim Care Respir J. 2013;22(4):439–48.
Singh D, Corradi M, Spinola M, et al. Extrafine beclometasone diproprionate/formoterol fumarate: a review of its effects in chronic obstructive pulmonary disease. NPJ Prim Care Respir Med. 2016;26:16030.
Kanniess F, Scuri M, Vezzoli S, et al. Extrafine beclomethasone/formoterol combination via a dry powder inhaler (NEXThaler®) or pMDI and beclomethasone monotherapy for maintenance of asthma control in adult patients: a randomised, double-blind trial. Pulm Pharmacol Ther. 2015;30:121–7.
Voshaar T, Spinola M, Linnane P, et al. Comparing usability of NEXThaler® with other inhaled corticosteroid/long-acting β 2-agonist fixed combination dry powder inhalers in asthma patients. J Aerosol Med Pulm Drug Deliv. 2014;27(5):363–70.
Sergio F, Chetta A, Pisi G, et al. A novel NEXT DPI® dry powder inhaler and its use in asthmatic and COPD population [abstract no. P831]. Eur Respir J. 2011;38(Suppl 55):152.
Scuri M, Alfieri V, Giorgio A, et al. Measurement of the inhalation profile through a novel dry powder inhaler (NEXThaler®) in asthmatic patients using acoustic monitoring [abstract no. A1931]. Am J Respir Crit Care Med. 2013;187.
Corradi M, Spinola M, Guasconi A, et al. Association of incident pneumonia and exacerbations with extrafine beclometasone dipropionate/formoterol (BDP/FF) in severe COPD patients: a post-hoc analysis of FORWARD study [abstract no. OA4831]. Eur Respir J. 2016;48.
Crisafulli E, Pisi R, Aiello M, et al. Prevalence of small-airway dysfunction among COPD patients with different GOLD stages and its role in the impact of disease. Respiration. 2017;93(1):32–41.
van den Berge M, ten Hacken NH, Cohen J, et al. Small airway disease in asthma and COPD: clinical implications. Chest. 2011;139(2):412–23.
Usmani OS, Singh D, Spinola M, et al. The prevalence of small airways disease in adult asthma: a systematic literature review. Respir Med. 2016;116:19–27.
Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi:10.1038/npjpcrm.2014.9.
Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin. 2009;25(8):2043–8.
Cazzola M, Beeh KM, Price D, et al. Assessing the clinical value of fast onset and sustained duration of action of long-acting bronchodilators for COPD. Pulm Pharmacol Ther. 2015;31:68–78.
Haughney J, Price D, Barnes NC, et al. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med. 2010;104(9):1237–45.
Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–71.
Ganderton D, Lewis D, Davies R, et al. Modulite®: a means of designing the aerosols generated by pressurized metered dose inhalers. Respir Med. 2002;96(Suppl D):S3–8.
Acknowledgements
The manuscript was reviewed by: F. Kanniess, Pulmonary Research Institute Hospital Grosshansdorf, Grosshansdorf, Germany; S. Saluja, Saran Ashram Hospital, Dayalbagh, Agra, India. During the peer review process, the manufacturer of extrafine beclometasone/formoterol FDC inhalers was offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
S. Dhillon is a salaried employee of Adis/Springer, is responsible for the article content and declares no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dhillon, S. Extrafine beclometasone dipropionate/formoterol fumarate metered-dose and dry-powder inhalers in asthma and chronic obstructive pulmonary disease: a profile of their use. Drugs Ther Perspect 33, 260–271 (2017). https://doi.org/10.1007/s40267-017-0397-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-017-0397-7