Abstract
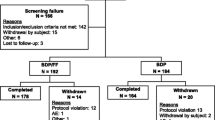
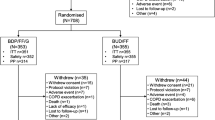
A new pediatric fixed combination of beclometasone dipropionate (BDP) 50 μg and formoterol fumarate (FF) 6 μg via pressurized metered-dose inhaler (pMDI) (CHF1535, Chiesi, Italy) was investigated. In a double-blind, randomized, placebo-controlled, cross-over study, a single CHF1535 administration using AeroChamber Plus™ spacer device (2 actuations, total dose BDP 100 μg/FF 12 μg) was compared to the same pMDI free combination in 56 asthmatic children aged ≥ 5 and < 12 years. Primary efficacy variable was forced expiratory volume during the first second (FEV1) area under the curve corrected by time over 12 h following morning dose (AUC0-12h). Further CHF1535 doses (50 μg/6 μg, 100 μg/12 μg, and 200 μg/24 μg) were also explored. Adverse events, electrocardiogram, and vital signs were monitored for safety. CHF1535 was non-inferior to free combination [adjusted mean difference (95% CI) 0.004 L (− 0.050, 0.041] with lower confidence limit greater than the limit set at 0.1 L. FEV1 AUC0-12h of each CHF1535 dose vs placebo were 0.037 L (p = 0.160), 0.119 L (p < 0.001), and 0.094 (p < 0.001) for 50/6, 100/12, and 200/24, respectively. No safety signals were found.
Conclusion: CHF1535 was as effective as free combination BDP/FF, with a trend towards a dose-related response. All treatments were safe.
Trial registration: ClinicalTrials.gov ID: NCT01584492
What is Known: •Inhaled pressurized metered-dose solutions (pMDI) are the preferred treatment for pediatric asthma. •Combination therapy of inhaled corticosteroids and long-acting β2- agonists is a well-established approach to control airway inflammation and airway obstruction also in pediatric patients. | |
What is New: •A novel pediatric pMDI fixed combination of beclomethasone dipropionate 50 μg and formoterol fumarate 6 μg (CHF 1535) was non-inferior to the free combination at the same dose in pulmonary function over the 12-h post-dose period in asthmatic children, with trend towards a dose-related response. |


Similar content being viewed by others
Data availability
Study data are available at the archives of the Sponsor Chiesi Farmaceutici S.p.A. (Parma, Italy) and in the Investigator Files at the participating sites according to ICH/GCP Guidelines.
Abbreviations
- AEs:
-
Adverse events
- ANCOVA:
-
Analysis of covariance
- AUC:
-
Area under curve
- BDP:
-
Beclometasone dipropionate
- ECG:
-
Electro-cardiogram
- FEV1 :
-
Forced expiratory volume in 1 s
- FF:
-
Fluticasone furoate
- GINA:
-
Global initiative for asthma
- HFA:
-
Hydro-fluoro-alkane
- ICS:
-
Inhaled corticosteroid
- ISMB:
-
Independent safety monitoring board
- ITT:
-
Intention-to-treat
- LABA:
-
Long-acting beta-2 agonist
- pMDI:
-
Pressurized metered-dose inhaler
- PP:
-
Per population
- SAEs:
-
Serious adverse events
- SD:
-
Standard deviation
References
Global Initiative for Asthma (2020) Global strategy for asthma management and prevention
De Benedictis FM, Selvaggio D (2003) Use of inhaler devices in paediatric asthma. Paediatr Drugs. 5(9):629–638
Janssens HM, Tiddens HA (2006) Aerosol therapy: the special needs of young children. Paediatr Respir Rev. 7(Suppl 1):S83–S85
Newman SP (2005) Principles of metered-dose inhaler design. Respir Care. 50(9):1177–1190
Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, de Boeck K (2002) Budesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatr Pulmonol. 34(5):342–350
Fabbri LM, Nicolini G, Olivieri D, Papi A (2008) Inhaled beclometasone dipropionate/formoterol extra-fine fixed combination in the treatment of asthma: evidence and future perspectives. Expert Opin Pharmacother. 9(3):479–490
Lalloo UG, Malolepszy J, Kozma D, Krofta K, Ankerst J, Johansen B, Thomson NC (2003) Budesonide and formoterol in a single inhaler improve asthma control compared with icreasing the dose of corticosteroid in adults with mild-to-moderate asthma. Chest. 123(5):1480–1487
Kips JC, O’Connor BJ, Inman BD, Svensson K, Pauwels RA, O’Btrne PM (2000) A long-term study of the antiinflammatory effect of low-dose budesonide plus formoterol versus high-dose budesonide in asthma. Am J Respir Crit Care Med. 161(3 Pt 1):996–1001
Pohunek P, Kuna P, Carlsen CJ, De Boeck K (2006) Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma. Pediatr Allergy Immunol 17:458–465. https://doi.org/10.1111/j.1399-3038.2006.00425.x
Miller MREA (2005) Standardisation of spirometry. Eur Respir J 26(2):319–338
Quanjer PH, Borsboom GJ, Brunekreef B, Zach M, Forche G, Cotes JE, Sanchis J, Paoletti P (1995) Spirometric reference values for white European children and adolescents: Polgar revisited. Pediatr Pulmonol. 19(2):135–142
Chuchalin AG, Manjra AI, Rozinova NN, Skopková O, Cioppa GD, Till D, Kaiser G, Fashola T, Kottakis J (2005) Formoterol delivered via a new multi-dose dry powder inhaler (Certihaler™) is as effective and well tolerated as the formoterol dry powder inhaler (Aerolizer®) in children with persistent asthma. J Aerosol Med 18(1):63–73
Pohunek P, Matulka M, Rybnicek O, Kopriva F, Honomichlova H, Svobodova T (2004) Dose-related efficacy and safety of formoterol (Oxis®) Turbuhaler® compared with salmeterol Diskhaler® in children with asthma. Paediatric Allergy Immunol 15(1):32–39
Durrington HJ, Farrow SN, Loudon AS, Ray DW (2014) The circadian clock and asthma. Thorax 69(1):90–92. https://doi.org/10.1136/thoraxjnl-2013-203482
Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg-Smith SM, Kline JN (2007) Placebo response in asthma: a robust and objective phenomenon. J Allergy Clin Immunol 119(6):1375–1381. https://doi.org/10.1016/j.jaci.2007.03.016
Acknowledgments
The authors would like to thank the patients and their families for their participation in the study as well as Chiesi Farmaceutici S.p.A. (Parma, Italy) for support in conducting this study; CROMSOURCE srl, Via Giorgio De Sandre 3, 37135 Verona (Italy) for the management and periodic monitoring of the clinical sites; BIOMEDICAL SYSTEMS, Waversesteenweg 1945 chaussée de Wavre/B, 1160 Brussels (Belgium), for centralized spirometry and ECG services; and CROS NT srl, Via Germania 2, 37135 Verona (Italy) for data management and statistical analysis. In addition to the authors, the following investigators participated in this clinical trial: Malgorzata Zuronska-Gebala, Specialistic Medical Centre, Tarnow (Poland) and Olga Yablon, Vinnytsya Regional Children Clinical Hospital, Department of Pulmonology, Department of Pediatry No. 1, Vinnytsya 21029 (Ukraine).
Funding
This study was funded funded by the Chiesi Farmaceutici S.p.A., Via Palermo 26/A, 43122 Parma (Italy).
Author information
Authors and Affiliations
Contributions
Petr Pohunek and Guido Varoli contributed to the study design and management and manuscript medical writing; Annamaria Muraro and Elena Carzana generated the random allocation sequence and supported the data management and statistical analysis; Silvia Armani contributed to the design of the study and study drug packaging and distribution. All the other authors acted as investigators and contributed to the management of the study and data collection. All the authors were involved in the review and editing of the draft manuscript and provided their approval of the final version of the manuscript. Petr Pohunek, the corresponding author, warrants that the manuscript is free of bias.
Corresponding author
Ethics declarations
Conflict of interest
Yuriy Reznichenko, Svetlana Mokia-Serbina, Jerzy Brzostek, Viktoriya Kostromina, Mykola Kaladze, and Jadwiga Kaczmarek all received clinical research funds from Chiesi Farmaceutici S.p.A. as site investigators for this study. Petr Pohunek received funds from Chiesi Farmaceutici S.p.A. as Scientific Coordinating Investigator of the study, lecturer fee from Novartis, TEVA, AstraZeneca and travel grant from Novartis and is a member of the advisory boards of Novartis and GlaxoSmithKline. Guido Varoli, Annamaria Muraro, Elena Carzana, and Silvia Armani are full employees of Chiesi Farmaceutici S.p.A., sponsor of the study.
Ethics approval
The regulatory authorities in each participating country and Ethical Review boards for each institution and prior to registration (ClinicalTrials.gov ID: NCT01584492) approved the study.
Consent to participate
Each patient, parents and/or legal guardian provided with the written informed consent prior to study entry.
Consent for publication
All the authors were involved in the review and editing of the draft manuscript and provided their approval of the final version of the manuscript and consent for its publication.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pohunek, P., Varoli, G., Reznichenko, Y. et al. Bronchodilating effects of a new beclometasone dipropionate plus formoterol fumarate formulation via pressurized metered-dose inhaler in asthmatic children: a double-blind, randomized, cross-over clinical study. Eur J Pediatr 180, 1467–1475 (2021). https://doi.org/10.1007/s00431-020-03888-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-020-03888-x