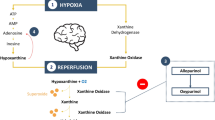
Abstract
Background
The CRUCIAL trial (NCT04217421) is investigating the effect of postnatal and perioperative administration of allopurinol on postoperative brain injury in neonates with critical congenital heart disease (CCHD) undergoing cardiac surgery with cardiopulmonary bypass (CPB) shortly after birth.
Objective
This study aimed to characterize the pharmacokinetics (PK) of allopurinol and oxypurinol during the preoperative, intraoperative, and postoperative phases in this population, and to evaluate target attainment of the current dosing strategy.
Methods
Nonlinear mixed-effects modeling was used to develop population PK models in 14 neonates from the CRUCIAL trial who received up to five intravenous allopurinol administrations throughout the postnatal and perioperative periods. Target attainment was defined as achieving an allopurinol concentration >2 mg/L in at least two-thirds of the patients during the first 24 h after birth and between the start and 36 h after cardiac surgery with CPB.
Results
A two-compartment model for allopurinol was connected to a one-compartment model for oxypurinol with an auto-inhibition effect on the conversion, which best described the PK. In a typical neonate weighing 3.5 kg who underwent cardiac surgery at a postnatal age (PNA) of 5.6 days, the clearance (CL) of allopurinol and oxypurinol at birth was 0.95 L/h (95% confidence interval 0.75–1.2) and 0.21 L/h (0.17–0.27), respectively, which subsequently increased with PNA to 2.97 L/h and 0.41 L/h, respectively, before CPB. During CPB, allopurinol and oxypurinol CL decreased to 1.38 L/h (0.9–1.87) and 0.12 L/h (0.05–0.22), respectively. Post-CPB, allopurinol CL increased to 2.21 L/h (1.74–2.83), while oxypurinol CL dropped to 0.05 L/h (0.01–0.1). Target attainment was 100%, 53.8%, and 100% at 24 h postnatally, 24 h after the start of CPB, and 36 h after the end of cardiac surgery, respectively. The combined concentrations of allopurinol and oxypurinol maintained ≥ 90% inhibition of xanthine oxidase (IC90XO) throughout the postnatal and perioperative period.
Conclusions
The minimal target concentration of allopurinol was not achieved at every predefined time interval in the CRUCIAL trial; however, the dosing strategy used was deemed adequate, since it yielded concentrations well exceeding the IC90XO. The decreased CL of both compounds during CPB suggests influence of the hypothermia, hemofiltration, and the potential sequestration of allopurinol in the circuit. The reduced CL of oxypurinol after CPB is likely attributable to impaired kidney function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The dosing strategy used in the CRUCIAL trial achieved adequate xanthine oxidase inhibition in > 90% of neonates with critical congenital heart disease undergoing cardiac surgery with cardiopulmonary bypass (CPB) during the postnatal and perioperative periods. |
Reduced clearance of allopurinol and oxypurinol found during the use of CPB is likely due to hypothermia, hemofiltration, and possible sequestration of allopurinol within the circuit. |
The substantial decline in postoperative oxypurinol clearance suggests that impaired renal function, induced by CPB, may have influenced its elimination. |
1 Introduction
Critical congenital heart disease (CCHD) affects 0.3% of newborns and necessitates cardiac surgery with cardiopulmonary bypass (CPB) within the first weeks of life for the majority of cases [1]. While up to 90% of neonates survive into adulthood, many experience long-term neurological deficits, such as motor, cognitive, and behavioral impairments [2, 3]. During the critical periods surrounding birth and cardiac surgery with CPB, neonates with CCHD are at risk of hypoxic-ischemic brain injury due to hemodynamic instability [4, 5]. To address this concern, the CRUCIAL trial (NCT04217421) is currently investigating the neuroprotective potential of postnatal and perioperative allopurinol administration in minimizing perioperative brain injury in neonates with CCHD [6].
In prior clinical studies in neonates, allopurinol has shown promise as a neuroprotective agent in mitigating the effects of hypoxia and reperfusion [7]. Following a hypoxic-ischemic event, cytotoxic oxygen radicals are generated during the reperfusion and reoxygenation phases, leading to neuronal cell death. This destructive process is mediated by the enzyme xanthine oxidase (XO) [8, 9]. Allopurinol and its active metabolite oxypurinol act as XO inhibitors, thus preventing the formation of reactive oxygen species [10]. Previously, early postnatal allopurinol administration in asphyxiated neonates was found to provide a full XO inhibition for at least 24 h [11, 12]. Moreover, allopurinol has beneficial effects on the inflammatory response following ischemia-reperfusion events, and is known to improve postoperative outcomes in patients following CPB. This includes reduced inotropic scores, duration of mechanical ventilation, as well as length of stay in the intensive care unit and hospital [13, 14].
Neonates with CCHD require cardiac surgery with the support of CPB soon after birth, which is often accompanied by fluid resuscitation, therapeutic hypothermia, and hemofiltration [15]. The use of CPB is therefore often associated with hemodilution and hypothermia and is known to cause a profound change in blood flow, oxygen delivery, and enzyme activity [15, 16]. Moreover, during the first few hours or days following neonatal cardiac surgery with CPB, common complications such as cardiac stunning and inflammation can lead to low cardiac output syndrome and acute kidney injury (AKI) [17,18,19]. These potential physiological changes and CPB-induced complications can collectively affect metabolic enzymes and drug protein binding, and impair renal or hepatic function, which may alter the pharmacokinetics (PK) of allopurinol (metabolized by XO) and oxypurinol (cleared renally) during and after CPB [20, 21]. Moreover, the CPB circuit has been shown to sequester molecules in a highly unpredictable manner, especially for lipophilic or highly protein bound drugs [22, 23]. This effect is unclear for allopurinol and oxypurinol, which are hydrophilic compounds with low protein binding.
The PK of allopurinol and oxypurinol have been studied in asphyxiated neonates receiving 5–20 mg/kg of allopurinol postnatally [11, 12]. In neonates with moderate-to-severe hypoxic-ischemic encephalopathy (HIE), an auto-inhibition effect on allopurinol metabolism by oxypurinol was found, which was attributed to the involvement of XO in the metabolic pathway of allopurinol [12]. In critically ill neonates undergoing CPB, substantial alterations in PK parameters have been observed in more than 70% of studied drugs, including an increase in volume of distribution (Vd) and variable effects on clearance (CL) [24, 25]. Therefore, adjustments in dose level or dosing interval are often required in this population. The specific effects of CPB on the PK of allopurinol and oxypurinol during neonatal cardiac surgery remain unclear. The aim of this study was therefore to characterize the PK of allopurinol and oxypurinol during the postnatal and perioperative period, encompassing the early postnatal, preoperative, intraoperative, and postoperative phases, in neonates with CCHD undergoing cardiac surgery with CPB using a population PK modeling approach. The final population PK model was further used to evaluate the target attainment of the CRUCIAL trial under the current dosing strategy, and propose alternative dosing strategies when appropriate.
2 Methods
2.1 Patient Population, Blood Sampling, and Bioanalysis
The CRUCIAL trial is a phase III, randomized, quadruple-blinded, placebo-controlled, multicenter trial primarily investigating the effect of early postnatal and perioperative allopurinol administration on postoperative brain injury in neonates with CCHD. Evaluation of the PK profile of allopurinol and oxypurinol in this population was one of the secondary objectives of the trial. Specific details of the study protocol have been published [6] and the trial is registered at www.clinicaltrials.gov (NCT04217421). While the trial is being conducted in four academic centers in The Netherlands, the PK substudy was performed exclusively at Wilhelmina Children’s Hospital, University Medical Center Utrecht. In this center, neonates with a prenatal diagnosis of CCHD who were anticipated to require cardiac surgery with CPB within their first month of life were included in the PK substudy. Ethical approval was granted by the national and institutional Medical Research Ethics Committees and Central Committee on Research Involving Human Subjects (NL62772.041.18; EudraCT 2017-004596-31), and written informed consent for study participation was obtained from all parents or legal guardians. Randomization was performed by Ace Pharmaceuticals in blocking (by Randlist version 1.2 software). In its design and analysis, all measures were taken to ensure sustained blinding of the cases included in the PK substudy.
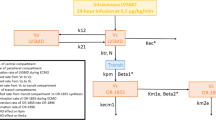
Schedules for dose administrations and blood samples for PK analysis are summarized in Fig. 1. In the intervention group, neonates diagnosed with CCHD before birth received five allopurinol administrations intravenously, with a dose of 20 mg/kg per administration: early postnatally within 45–60 min after birth (DOSE 1) and 12 h after the first dose (DOSE 2), preoperatively 12 h prior to cardiac surgery (DOSE 3), intraoperatively at the start of CPB (DOSE 4), and postoperatively 24 h after surgery (DOSE 5). To minimize the burden on the neonates, we applied a sparse blood sampling approach, limiting each sample to 0.5 mL. In addition, we combined blood sampling for this study as much as possible with clinically indicated blood draws. Samples were collected via arterial, venous, or capillary access. If there was no arterial or venous access, capillary blood sampling was exclusively performed when clinically warranted.
Dosing and blood sampling schedule for pharmacokinetic analysis [6]. Sample A was intended for measuring biomarkers and was excluded from the analysis. CPB cardiopulmonary bypass
Plasma concentrations of allopurinol and oxypurinol were quantified by The Ardena Bioanalytical Laboratory in accordance with Good Clinical Practice guidelines. The applied assay was validated according to the European Medicines Agency guidelines (International Conference on Harmonisation 2012). Allopurinol and oxypurinol in human plasma samples were extracted by protein precipitation using ethanol. After precipitation and centrifugation, the supernatant was injected into the liquid chromatography-tandem mass spectrometry (LC–MS/MS) chromatographic system for the determination of allopurinol and oxypurinol. The chromatographic separation was performed on an Astec® CHIROBIOTIC®V HPLC column using isocratic elution. An API 4000 tandem mass spectrometer equipped with a turbo ion spray probe operating in the negative multiple reaction monitoring mode was used for quantification. The lower limit of quantification (LLOQ) was 0.05 mg/L for allopurinol and 0.0467 mg/L for oxypurinol. Accuracy and precision of all quality control samples met the predefined acceptance criteria.
2.2 Structural Model Development
Due to the expected pronounced impact of CPB on blood flow and fluid balance, a population PK model was developed in three sequential steps. The first step involved analyzing the PK of allopurinol and oxypurinol during the postnatal-preoperative period, followed by the intraoperative period, and finally the postoperative period. The three periods were divided by the start and end times of CPB. A table describing overall model development is provided in the electronic supplementary material (ESM).
As a starting point, our previously developed model describing allopurinol and oxypurinol in neonates with HIE was used as the base structural model (see the ESM) [11, 12]. This model consisted of two sequential one-compartment models with an auto-inhibition effect on allopurinol metabolism by oxypurinol. The CL and Vd of allopurinol and oxypurinol during the postnatal-preoperative period (CLPostnatal and VdPostnatal), as well as the effect of auto-inhibition, were estimated and were compared with the estimations in HIE neonates.
During the intraoperative period, an alteration in the CL and Vd of allopurinol and oxypurinol was anticipated. Besides several physiological changes during CPB, alterations in CL and Vd might also be attributed to the influence of the CPB circuit. To account for the impact of fluctuating blood flow and the potential for drug sequestration within the CPB circuits on the PK of allopurinol and oxypurinol, separate CL and Vd (CLCPB and VdCPB) were estimated during this period using Eqs. (1) and (2).
where ECL,CPB and EVd,CPB represent the fractional changes in CL and Vd compared with the values at the postnatal-preoperative period, respectively.
In the postoperative period, changes in PK were expected to be attributable to various factors related to the cessation of CPB, the change in hemodynamics, and the altered anatomical conditions (e.g., cardiac stunning), which could lead to potential impairment of renal or hepatic function. Therefore, to characterize the PK of allopurinol and oxypurinol during this period, an additional set of CL and Vd values were estimated using Eqs. (3) and (4).
where the fractional changes in CL and Vd compared with the values at the postnatal-preoperative period were estimated, denoted as ECL,Postop and EVd,Postop, respectively.
2.3 Covariate Model Development
Parameters related to CL and Vd were modeled with body weight-based allometric scaling, using fixed power exponents of 0.75 and 1 for CL and Vd, respectively, and were normalized to a body weight of 3.5 kg to compare with previous models in neonates with HIE [11, 12]. In most critically ill neonates, a gradual recovery of organ function and cardiac output after birth could be expected due to the stabilization under the setting of pediatric intensive care [26]. Therefore, during the postnatal-preoperative period, the effect of recovery on allopurinol and oxypurinol CLpostnatal were evaluated using postnatal age (PNA) as an indicator, testing by a linear function (Eq. 5) and a sigmoidal Hill function (Eq. 6). In Eq. (6), TM50 is the PNA at which the recovery effect reaches 50% of the maximum value and the Hill coefficient describing the slope of the sigmoidal curve.
2.4 Pharmacokinetic Data Analysis
Population PK analysis was conducted using NONMEM (version 7.5; ICON Development Solutions, Ellicott City, MD, USA) [27], with support from Perl-speaks-NONMEM (PsN, version 5.0) [28] and Pirana (version 2.9.9) [29].
The first-order conditional estimation method with interaction (FOCE-I) option was employed to estimate PK parameters and their variability. Individual PK parameters were estimated using the maximum a posteriori Bayesian estimation method with the POSTHOC option in NONMEM.
Between-subject variability (BSV) was assessed using an exponential variance model assuming a log‐normal distribution (Eq. 7). Residual unexplained variability was evaluated with a proportional error model, an additive error model, or a combined proportional and additive model (Eq. 8).
where \(P_{{{\text{pop}}}}\) is the population estimate for a parameter, \({{P}}_{i}\) the individual value for that parameter, and \(\eta_{i}\) is the normally distributed between‐subject variability of the \(i{\text{th}}\) individual with a mean of zero and a variance of \(\omega^{2}\). \(Y_{i,j }\) is the observed concentration and \(C_{i, j}\) is the predicted concentration for the \(j{\text{th}}\) observation of the \(i{\text{th}}\) individual. \(\varepsilon_{1}\) and \(\varepsilon_{2}\) are the proportional and additive residual errors, respectively, which are normally distributed with a mean of zero and a variance of \(\sigma^{2}\).
2.5 Exposure and Target Attainment
The predefined objective of the CRUCIAL PK substudy was to attain a target concentration of allopurinol > 2 mg/L in at least two-thirds (> 66%) of the patients, postnatally within approximately the first 24 h after birth, defined as the time period between DOSE 1 and 12 h after DOSE 2, and perioperatively between the start of cardiac surgery with CPB and 36 h after cardiac surgery with CPB, defined as the time period between DOSE 4 and 12 h after DOSE 5.
The predefined target concentration of allopurinol was chosen based on previous studies showing that allopurinol effectively inhibits XO at plasma concentrations exceeding 2 mg/L [30, 31]. We aimed to achieve this target concentration in more than 66% of the analyzed cases. This objective was outlined in the CRUCIAL study protocol [6], aligning with a recent report on neonates with HIE receiving allopurinol [11], and following guidance of the European Medicines Agency (scientific advice and protocol assistance).
To assess the effect of the current allopurinol dosing regimen used in the CRUCIAL trial, three PK targets were evaluated. First, as defined in the trial’s protocol, allopurinol concentrations 12 h after each dose administration and 24 h after DOSE 2, DOSE 4 and DOSE 5 were derived from the final PK models. Target attainment of allopurinol concentration > 2 mg/L were calculated. Second, the concentrations of allopurinol plus oxypurinol at the aforementioned timepoints were compared with a target concentration of 6.22 mg/L, which corresponds to 90% of maximal XO inhibition (IC90XO), deriving from our previous study in neonates with HIE [11]. Lastly, the area under the plasma concentration-time curve (AUC) for 12 h after each dose was calculated using the individual MAP Bayesian parameter estimates of the final PK models, and were used to compare allopurinol and oxypurinol exposures in neonates with CCHD and those with moderate-to-severe HIE (ALBINO trial) [32]. In neonates with HIE receiving early postnatal allopurinol administration, the median allopurinol and oxypurinol AUC12 were 129 mg/L*h (interquartile range 93.6–161) and 42.7 mg/L*h (29.4–59.3), respectively [12].
2.6 Model Evaluation
Model adequacy was guided by physiological plausibility, statistical significance, and graphical evaluation. The change in objective function value (OFV), which equals minus two times the log-likelihood, was used to define statistical significance between hierarchical models following a Chi-square distribution. A decrease in OFV of > 3.84 for 1 degree of freedom, representing a p value of < 0.05, was considered statistically significant. Akaike information criterion (AIC) was used to compare the model fit between non-hierarchical models, and goodness-of-fit plots were used to assist graphical evaluation using R (version 4.0) and Xpose (version 4). A visual predictive check (VPC) [33] and sampling importance resampling (SIR) [34] were performed to assess the predictive performance and parameter precision with 95% confidence intervals (CIs) for the final model.
3 Results
3.1 Patient Characteristics
Allopurinol and oxypurinol data from 14 neonates with CCHD were used for the PK analysis. In one neonate, the cardiac surgery was performed in a non-participating center; thus, the PK samples of this neonate were only available for the postnatal stage. Thirteen neonates underwent neonatal cardiac surgery at Wilhelmina Children’s Hospital Utrecht with the support of CPB starting at a median PNA of 5.6 days. The median total duration of the cardiac surgery was 320 min. Clinical characteristics of the included study population are summarized in Table 1. The number of analyzed allopurinol and oxypurinol observations during the postnatal-preoperative, intraoperative, and postoperative periods was 60, 36, and 44, respectively. Only 5.8% of the measured allopurinol concentrations were below the LLOQ, and were therefore included as LLOQ/2, with an additive residual error component of LLOQ/2 fixed to the error model. The observed concentrations of allopurinol and oxypurinol are plotted against the sampling time point in Fig. 2.
Allopurinol and oxypurinol concentrations versus numeral sampling time point. A description of sampling time points is provided in Fig. 1
3.2 Model Development
The PK of allopurinol and oxypurinol were best described by a two-compartment model and a subsequent one-compartment model with an auto-inhibition effect on allopurinol metabolism by oxypurinol. The parameter estimates of the final model are summarized in Table 2. A two-compartmental model was required to capture the maximal allopurinol concentrations measured at 10 min after the onset of CPB, in which a very small central compartment (V1) of 0.1 L and a large inter-compartment CL (Q1) of 6.79 L/h were estimated. The AIC values were 839 for the one-compartment model and 736 for the two-compartment model, supporting the need for a two-compartment model. This additional fast distribution process was likely an artifact of the very short sampling interval in the intraoperative period. Therefore, V1 and Q1 were fixed during the model building process to provide an adequate model fit.
Following our previous model based on neonates with HIE, the metabolism of allopurinol was more accurately described by an auto-inhibition effect driven by oxypurinol. The effect size of the auto-inhibition was estimated based on the data collected during the early postnatal period (PNA ≤ 3 days), in which the half-maximum effect was reached with an oxypurinol concentration (IC50auto-inhibition) of 1.1 mg/L. The IC50auto-inhibition value was fixed during the latter model development stages to improve model stability and to allow further covariate analysis.
During the early postnatal period, the CLPostnatal of allopurinol and oxypurinol were comparable with neonates with HIE. Including PNA as a covariate on the CLPostnatal of allopurinol and oxypurinol significantly improved the model fit, with a dOFV of − 80 with 2 degrees of freedom (p < 0.01), suggesting an impact of recovery on drug CL after birth. This recovery effect was empirically described by a sigmoidal Hill equation, with a TM50 of PNA 4.2 days and a Hill coefficient of 2.98. The maximal increase in allopurinol and oxypurinol CLPostnatal until the start of cardiac surgery (3- to 14-day interval) were 3-fold and 1.35-fold, respectively. The effect of recovery was fixed to the identified values for further model development. In Fig. 3, the CLs in a typical neonate (3.5 kg) who underwent cardiac surgery at a PNA of 5.6 days are illustrated. At birth, the CLPostnatal of allopurinol and oxypurinol were 0.95 L/h and 0.21 L/h, respectively; while before the onset of CPB, the CLPostnatal increased to 2.97 L/h and 0.41 L/h, respectively. During the period of cardiac surgery with CPB, the CLCPB of allopurinol and oxypurinol decreased to 1.38 L/h and 0.12 L/h, respectively. Consequently, there was minimal fluctuation in oxypurinol concentrations during the onset of CPB (Fig. 2, samples L, M, and N). After the cessation of CPB, the CLPostop of allopurinol increased to 2.21 L/h, while CLPostop of oxypurinol dropped to 0.05 L/h, most likely due to AKI, which is a common complication of CPB [17,18,19].
The clearance in a typical neonate (3.5 kg) over postnatal age, starting cardiac surgery with cardiopulmonary bypass at a postnatal age of 5.6 days. At birth, the clearance of allopurinol and oxypurinol was 0.95 L/h and 0.21 L/h, respectively, and increased to 2.97 L/h and 0.41 L/h before the start of CPB, respectively. During the period of cardiac surgery with CPB (shaded area), the clearance of allopurinol and oxypurinol decreased to 1.38 L/h and 0.12 L/h, respectively. After CPB, the clearance of allopurinol increased to 2.21 L/h, while clearance of oxypurinol dropped to 0.05 L/h. CPB cardiopulmonary bypass
The Vd of allopurinol and oxypurinol in neonates with CCHD was 2.22 L and 12 L at birth, respectively, similar to the values found in neonates with HIE. Throughout the perioperative period, including the intraoperative and postoperative stages, the Vd of allopurinol and oxypurinol gradually increased by 42% to 3.16 L and 48% to 18 L, respectively.
BSV in the CL of allopurinol and the Vd of oxypurinol across all periods were estimated at 36% and 42%, respectively. Between-occasion variability was estimated for the CL of allopurinol and oxypurinol between stages. The final model provided an adequate description of allopurinol and oxypurinol observations in neonates with CCHD during the postnatal-preoperative, intraoperative, and postoperative periods. Goodness-of-fit plots (Fig. 4) and the VPC (Fig. 5) based on the final model showed no major deviations. The precision of the parameter estimates in the final model were confirmed by SIR analysis.
Prediction-corrected visual predictive check of allopurinol and oxypurinol based on the final model. a Postnatal period; x-axis represents the time (in hours) after birth. b Perioperative period, including the preoperative, intraoperative and postoperative phases; x-axis represents the time (in hours) after the third dose (samples J–R). Black solid and dashed lines represent the median and 80% interval of the prediction-corrected observations; red shaded area represents the 95% CI of the median prediction; and blue shaded area represents the 95% CI of the 10th and 90th prediction intervals. Automatic binning was used, with the number of bins set to four for the postnatal period and five for the perioperative period. CI confidence interval
3.3 Exposure and Target Attainment
The minimal target concentrations of allopurinol > 2 mg/L were reached in 100%, 53.8%, and 100% of patients at 24 h after birth, 24 h after the start of CPB, and 36 h after the end of cardiac surgery, respectively (Table 3). Although the predefined target of allopurinol concentration > 2 mg/L was not reached in > 66% of patients at 24 h after the start of CPB, dosing adjustments were considered not needed since allopurinol plus oxypurinol concentrations achieved IC90XO of 6.22 mg/L in >90% of patients at this time point, suggesting sufficient XO inhibition (Table 3 and Fig. 6) [11]. Allopurinol and oxypurinol AUC12 after each dose are shown in Fig. 7. The AUC12 of allopurinol and oxypurinol throughout the whole observation period was comparable with, or higher than, the exposures in neonates with HIE [12].
Allopurinol plus oxypurinol concentrations over time after birth or time after the start of cardiac surgery compared with the IC90 value of 6.22 mg/L for xanthine oxidase inhibition [11]. The dotted line corresponds to 72 h after birth. IC90 90% inhibitory concentration
Allopurinol and oxypurinol exposures compared with neonates with moderate-to-severe hypoxic-ischemic encephalopathy (ALBINO trial) [32]. Shaded areas represent the IQR of allopurinol and oxypurinol AUC12 in neonates from the ALBINO study, in which the median (IQR) was 129 mg/L*h (93.6–161) and 42.7 mg/L*h (29.4–59.3), respectively [12]. IQR interquartile range, AUC12 area under the plasma concentration-time curve from time zero to 12 h
4 Discussion
This study aimed to evaluate the current dosing strategy of early postnatal and perioperative allopurinol administration in neonates undergoing cardiac surgery with CPB, as applied in the CRUCIAL trial, using a population PK model for allopurinol and oxypurinol. The predefined PK targets were to achieve allopurinol concentrations > 2 mg/L in more than 66% of patients during the first 24 h after birth and between the start of cardiac surgery and 36 h after cardiac surgery with CPB.
Our previous studies in neonates with moderate-to-severe HIE suggested that allopurinol and oxypurinol provided 90% XO inhibition with a combined concentration of 6.22 mg/L [11]. In the current study, this IC90XO target was achieved in > 70% of analyzed neonates within 12–24 h after each dose. These results suggest that the current dosing strategy was sufficient in maintaining drug levels that provide adequate XO inhibition throughout the postnatal and perioperative time windows (Table 3 and Fig. 6). Therefore, although the predefined minimal allopurinol target concentration was not always achieved, the current dosing strategy employed in the CRUCIAL trial was considered sufficient. The effect of oxypurinol on XO inhibition was not taken into account for the definition of the target concentration in the clinical trial protocol [6]. It is known that oxypurinol inhibits XO as effectively as allopurinol and has a much longer half-life, which therefore is believed to be the primary active moiety responsible for its pharmacological effects [35].
Significant changes in the CL and Vd of allopurinol and oxypurinol among the postnatal, intraoperative, and postoperative phases were characterized in this population. Between birth and the start of cardiac surgery with CPB, the CLPostnatal of allopurinol and oxypurinol was found to increase with PNA. The maximal increase in CLPostnatal was threefold for allopurinol and 1.35-fold for oxypurinol up until 1.5 weeks of life, in which the half-maximum value was reached at 4.2 days of life. In critically ill neonates, several drugs predominantly cleared renally or hepatically have shown a strong increase in CL after birth [26, 36]. While this increase is likely partly due to maturation, it cannot be attributed solely to maturation effects [26, 37]. Previous studies suggested that the supportive and stabilizing environment of intensive care may play an important role in the observed increase in drug CL [26]. Such an effect is very likely also present in our study population, which may explain the increasing CLPostnatal of allopurinol and oxypurinol. Nevertheless, as we lack data from critically ill and non-critically ill neonates administered the same drug, the effect of maturation and recovery from illness cannot be distinguished.
In addition to the effects of recovery, the substantial increase in allopurinol CLPostnatal found in neonates with CCHD may also be related to the development of hypoxic-ischemic events during the postnatal period [38]. Unlike neonates with HIE who experience severe fetal hypoxia, neonates with CCHD typically encounter milder hypoxic-ischemic events after birth. These events can be attributed to the cardiac defect itself, which alters blood flow and oxygen delivery, or may result from hemodynamic instability during the delivery process or therapeutic interventions (e.g., balloon atrial septostomy) [4]. A hypoxic-ischemic event is known to increase the level of highly activated XO as a consequence of primary energy failure [39]. In neonates with CCHD, the level of activated XO was likely lower at birth when compared with neonates with HIE, while elevated during the postnatal period, which explains the threefold increase in allopurinol CL in this period.
In this study, the median total duration of the cardiac surgery with CPB was 320 min. When compared with the PK parameters estimated before the start of CPB in a typical neonate with a PNA of 5.4 days, the CLCPB of allopurinol and oxypurinol decreased by 54% and 71%, respectively, and VdCPB increased by 47% and 33%, respectively. In this study, neonates underwent therapeutic hypothermia during cardiac surgery, with the lowest rectal temperature during CPB measured at a median of 27.7 °C. The decreased CLCPB can be linked to the reduced enzyme activity resulting from the application of therapeutic hypothermia [16]. Additionally, the use of a CPB may reduce blood flow to vital metabolic organs such as liver and kidneys, subsequently causing a decrease in drug CL [15]. The increased VdCPB could be a consequence of hemodilution resulting from fluid support required to maintain circulation throughout the surgery with CPB [20, 21]. Moreover, since both allopurinol and oxypurinol are hydrophilic drugs, factors such as the additional volume required for the CPB device and those related to the CPB procedure (e.g., capillary leakage) could also contribute to the increased Vd.
Of note, during the CPB period, a decrease in allopurinol concentrations was observed, while the oxypurinol concentrations remained stable at lower values (Fig. 2, samples L, M, and N) when compared with the last observation before the surgery (Fig. 2, sample K). This result suggested that allopurinol was likely not fully converted to oxypurinol during the CPB period, which may be attributed to the use of conventional ultrafiltration during CPB or CPB sequestration. Nevertheless, a full conversion of allopurinol to oxypurinol was assumed in this analysis due to limited data. Conventional ultrafiltration is frequently utilized during CPB to mitigate the consequences of hemodilution and anemia by removing intravascular plasma water [40]. Consequently, conventional ultrafiltration might have a significant role in drug CL during CPB, which warrants additional investigation. Although the CPB circuit is more likely to retain drugs that are highly lipophilic and protein-bound, which is not the case for allopurinol and oxypurinol, the potential allopurinol loss in the circuit cannot be ruled out [22, 23]. In a previous study, hypoxanthine, which is a structural analog of allopurinol, was found to accumulate in isolated extracorporeal membrane oxygenation circuits over time during bypass [41].
A significant reduction in postoperative oxypurinol CL was found, dropping to 0.05 L/h, which was even lower than the 0.21 L/h observed shortly after birth (Table 2). This decreased CL led to a rapid increase in oxypurinol exposure (Fig. 7), potentially providing additional cerebral protection to neonates with CCHD following surgery with CPB. The decline in oxypurinol CL is likely attributed to impaired renal function caused by AKI, which is a common complication that can arise after surgery with CPB. Ischemia-reperfusion injury is one of its major causes, which occurs when the kidneys experience reduced blood flow during CPB, followed by reperfusion, resulting in kidney damage [17, 18]. Furthermore, an immediate recovery of allopurinol CL was observed after CPB. This increase in allopurinol CL might be related to the cessation of CPB, which is known to induce hypoxia and subsequently elevate XO activity [5].
To capture the maximal allopurinol concentrations measured 10 min after the onset of CPB, a very small central compartment and a large intercompartment CL were assumed. This approach was necessary due to the intensive sampling schedule during the CPB period (10 and 30 min post-dose) compared with other periods (1–3 h post-dose). Therefore, these parameter settings were included to improve model fit and stability, despite lacking pharmacological and physiological interpretation.
5 Conclusion
This study represents the first characterization of the PK of allopurinol and oxypurinol in neonates with CCHD undergoing cardiac surgery with the support of CPB throughout the postnatal and perioperative periods. The CL of both compounds increased significantly after birth, which may be attributed to organ recovery during stabilization in the intensive care unit or the progression of hypoxemia resulting from multiple mild hypoxic-ischemic insults due to hemodynamic instability associated with the cardiac defect. Changes in drug CL and Vd with the use of CPB were quantified and a potential loss of allopurinol was revealed, underlying a mix effect associated with hypothermia, hemodilution, hemofiltration, and sequestration by the CPB circuit. Furthermore, a decrease in oxypurinol CL was observed, likely indicating impaired kidney function following CPB. This reduced CL led to higher oxypurinol exposure, which could potentially enhance cerebral protection. Most importantly, plasma concentrations of allopurinol and oxypurinol were maintained at sufficient concentrations to inhibit XO, thereby rendering dose adjustment unnecessary.
References
Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900.
Latal B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin Perinatol. 2016;43:173–85.
Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5):e1502–8.
Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–23.
Stegeman R, Feldmann M, Claessens NHP, Jansen NJG, Breur JMPJ, de Vries LS, et al. A uniform description of perioperative brain MRI findings in infants with severe congenital heart disease: results of a European Collaboration. AJNR Am J Neuroradiol. 2021;42:2034–9.
Stegeman R, Nijman M, Breur JMPJ, Groenendaal F, Haas F, Derks JB, et al. CeRebrUm and CardIac Protection with ALlopurinol in Neonates with Critical Congenital Heart Disease Requiring Cardiac Surgery with Cardiopulmonary Bypass (CRUCIAL): study protocol of a phase III, randomized, quadruple-blinded, placebo-controlled, Dutch mu. Trials. 2022. https://doi.org/10.1186/s13063-022-06098-y.
Annink KV, Franz AR, Derks JB, Rüdiger M, van Bel F, Benders MJNL. Allopurinol: old drug, new indication in neonates? Curr Pharm Des. 2018;23:5935–42.
Ozsurekci Y, Aykac K. Oxidative stress related diseases in newborns. Oxid Med Cell Longev. 2016;2016:1–9.
Taverne YJHJ, Bogers AJJC, Duncker DJ, Merkus D. Reactive oxygen species and the cardiovascular system. Oxid Med Cell Longev. 2013;2013: 862423.
Day RO, Graham GG, Hicks M, McLachlan AJ, Stocker SL, Williams KM. Clinical pharmacokinetics and pharmacodynamics of allopurinol and oxypurinol. Clin Pharmacokinet. 2007;46:623–44.
Chu WY, Allegaert K, Dorlo TPC, Huitema ADR, Franz AR, Rüdiger M, et al. Semi-mechanistic modeling of hypoxanthine, xanthine, and uric acid metabolism in asphyxiated neonates. Clin Pharmacokinet. 2022;61:1545–58.
Chu WY, Annink KV, Nijstad AL, Maiwald CA, Schroth M, el Bakkali L, et al. Pharmacokinetic/pharmacodynamic modelling of allopurinol, its active metabolite oxypurinol, and biomarkers hypoxanthine, xanthine and uric acid in hypoxic-ischemic encephalopathy neonates. Clin Pharmacokinet. 2022;61:321–33.
Stegeman R, Lamur KD, van den Hoogen A, Breur JMPJ, Groenendaal F, Jansen NJG, et al. Neuroprotective drugs in infants with severe congenital heart disease: a systematic review. Front Neurol. 2018. https://doi.org/10.3389/fneur.2018.00521.
Talwar S, Selvam MS, Makhija N, Lakshmy R, Choudhary SK, Sreenivas V, et al. Effect of administration of allopurinol on postoperative outcomes in patients undergoing intracardiac repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2018;155:335–43.
Sarkar M, Prabhu V. Basics of cardiopulmonary bypass. Indian J Anaesth. 2017;61:760–7.
Pokorna P, Wildschut ED, Vobruba V, van den Anker J, Tibboel D. The impact of hypothermia on the pharmacokinetics of drugs used in neonates and young infants. Curr Pharm Des. 2015;21:5705–24.
Beken S, Akbulut BB, Albayrak E, Güner B, Ünlü Y, Temur B, et al. Evaluation of neonatal acute kidney injury after critical congenital heart disease surgery. Pediatr Nephrol. 2021;36:1923–9.
Van den Eynde J, Delpire B, Jacquemyn X, Pardi I, Rotbi H, Gewillig M, et al. Risk factors for acute kidney injury after pediatric cardiac surgery: a meta-analysis. Pediatr Nephrol. 2022;37:509–19.
Alten JA, Cooper DS, Blinder JJ, Selewski DT, Tabbutt S, Sasaki J, et al. Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med (United States). 2021;49:e941–51.
Holley FO, Ponganis KV, Stanski DR. Effect of cardiopulmonary bypass on the pharmacokinetics of drugs. Clin Pharmacokinet. 1982;7:234–51.
Buylaert WA, Herregods LL, Mortier EP, Bogaert MG. Cardiopulmonary bypass and the pharmacokinetics of drugs: an update. Clin Pharmacokinet. 1989;17:10–26.
Wildschut ED, Ahsman MJ, Allegaert K, Mathot RAA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109–16.
O’Hanlon CJ, Hannam JA, Anderson B, Holford N. A framework for drug pharmacokinetics during cardiopulmonary bypass. PAGE 31 (2023) Abstr 10427 [http://www.page-meeting.org/?abstract=10427. Accessed 05 Aug 2024
Yalcin N, Sürmelioglu N, Allegaert K. Population pharmacokinetics in critically ill neonates and infants undergoing extracorporeal membrane oxygenation: a literature review. BMJ Paediatr Open. 2022;6:e001512.
Sutiman N, Koh JC, Watt K, Hornik C, Murphy B, Chan YH, et al. Pharmacokinetics alterations in critically ill pediatric patients on extracorporeal membrane oxygenation: a systematic review. Front Pediatr. 2020;8:260.
Favié LMA, de Haan TR, Bijleveld YA, Rademaker CMA, Egberts TCG, Nuytemans DHGM, et al. Prediction of drug exposure in critically ill encephalopathic neonates treated with therapeutic hypothermia based on a pooled population pharmacokinetic analysis of seven drugs and five metabolites. Clin Pharmacol Ther Clin Pharmacol Ther. 2020;108:1098–106.
Beal S, Sheiner L, Boeckmann A, Bauer R. NONMEM users guides. ICON Dev. Solut. 2013.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)–a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85–94.
Keizer RJ, van Benten M, Beijnen JH, Schellens JHM, Huitema ADR. Piraña and PCluster: A modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed. 2011;101:72–9.
Kaandorp JJ, van den Broek MPH, Benders MJNL, Oudijk MA, Porath MM, Bambang Oetomo S, et al. Rapid target allopurinol concentrations in the hypoxic fetus after maternal administration during labour. Arch Dis Child Fetal Neonatal Ed. 2014;99:F144–8.
Kane AD, Camm EJ, Richter HG, Lusby C, Tijsseling D, Kaandorp JJ, et al. Maternal-to-fetal allopurinol transfer and xanthine oxidase suppression in the late gestation pregnant rat. Physiol Rep (United States). 2013;1:e00156.
Maiwald CA, Annink KV, Rüdiger M, Benders MJNL, Van Bel F, Allegaert K, et al. Effect of allopurinol in addition to hypothermia treatment in neonates for hypoxic-ischemic brain injury on neurocognitive outcome (ALBINO): study protocol of a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III). BMC Pediatr. 2019;19:210.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143.
Dosne AG, Bergstrand M, Karlsson MO. An automated sampling importance resampling procedure for estimating parameter uncertainty. J Pharmacokinet Pharmacodyn. 2017;44:509–20.
Spector T. Oxypurinol as an inhibitor of xanthine oxidase-catalyzed production of superoxide radical. Biochem Pharmacol. 1988;37:349–52.
Bilbao-Meseguer I, Rodríguez-Gascón A, Barrasa H, Isla A, Solinís MÁ. Augmented renal clearance in critically ill patients: a systematic review. Clin Pharmacokinet. 2018;57:1107–21.
Holford N, O’Hanlon CJ, Allegaert K, Anderson B, Falcão A, Simon N, et al. A physiological approach to renal clearance: from premature neonates to adults. Br J Clin Pharmacol. 2024;90:1066–80.
Bonthrone AF, Stegeman R, Feldmann M, Claessens NHP, Nijman M, Jansen NJG, et al. Risk factors for perioperative brain lesions in infants with congenital heart disease: A European Collaboration. Stroke. 2022;53:3652–61.
Epstein FH, McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–63.
Wang S, Palanzo D, Ündar A. Current ultrafiltration techniques before, during and after pediatric cardiopulmonary bypass procedures. Perfusion. 2012;27:438–46.
Marro PJ, Baumgart S, Delivoria-Papadopoulos M, Zirin S, Corcoran L, McGaurn SP, et al. Purine metabolism and inhibition of xanthine oxidase in severely hypoxic neonates going onto extracorporeal membrane oxygenation. Pediatr Res. 1997;41(4 Pt 1):513–20.
Acknowledgements
The authors thank the families who participated in this study, and also the data managers, independent experts, Data Monitoring Committee, Ace Pharmaceuticals (Zeewolde, The Netherlands), and monitor for their close involvement in the study. In addition, the authors would like to thank the Congenital Heart Disease LifeSpan Study group and clinical staff from the Departments of Obstetrics, Neonatology, Pediatric Cardiology, Pediatric Intensive Care, Anesthesiology, Congenital Cardiothoracic Surgery, Radiology, Child Development and Exercise Center, Medical Psychology, and Social Work in Erasmus Medical Center Rotterdam, University Medical Center Groningen, Radboud University Medical Center Nijmegen, and University Medical Center Utrecht. Furthermore, Karel Allegaert leads Work Package 8 (Pharmacokinetics) of the ALBINO trial (H2020-PHC-2015-two-stage, grant 667224) and acknowledges the ALBINO study group. Lastly, the authors thank all members of the CRUCIAL trial consortium for their contributions to this study. The CRUCIAL trial consortium also consists of: Academic Center for Congenital Heart Disease in Erasmus Medical Center Rotterdam/Radboud University Medical Center, Nijmegen: Ingrid M. van Beynum, Floris E. Udink ten Cate, Willem A. Helbing, Yannick J.H.J. Taverne, Willem P. de Boode, Ad J.C.C. Bogers, Koen F.M. Joosten, Pieter C. van de Woestijne, Inge I. de Liefde, Antony van Dijk, Natasja I.F. Meijer, Sinno H.P. Simons, Robin van der Lee, Jérôme M.J. Cornette, Neeltje E.M. van Haren. University Medical Center Groningen: Arend F. Bos, Rolf M.F. Berger, Ryan E. Accord, Sara C. Arrigoni, Leonie K. Duin, Martin C.J. Kneyber, Elisabeth M.W. Kooi , Joost M.A.A. van der Maaten, Linda C. Meiners, Mirthe J. Mebius, Gideon J. du Marchie Sarvaas, Ward Y. Vanagt. University Medical Center Utrecht: Nathalie H.P. Claessens, Bram van Wijk, Paul H. Schoof, Hanna Talacua, Trinette J. Steenhuis, Henriette ter Heide, Gabrielle G. van Iperen, Rian Bosch, Floris Groenendaal, Jan B. Derks, Roel de Heus, Mireille N. Bekker, Roelie M. Wösten-van Asperen, and Nicole van Belle-van Haaren. Biostatistics Julius Center: Daniela Cianci, Stavros Nikolakopoulos, Kit C.B. Roes. Central Pharmacy: Arief Lalmohamed, Karin Rademaker. Health Economic Evaluation: G. Ardine de Wit.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
The CRUCIAL study is funded by ZonMw, as part of the Goed Gebruik Geneesmiddelen, Grote Trials Ronde II program (https://www.zonmw.nl/en/; project number 848042002), the Hartekind Foundation (https://www.hartekind.nl), and Friends of the Wilhelmina Children’s Hospital Foundation (https://vriendenumcutrecht-wkz.nl).
Conflict of interest
Wan-Yu Chu, Maaike Nijman, Raymond Stegeman, Johannes M. P. J. Breur, Nicolaas J.G. Jansen, Joppe Nijman, Kim van Loon, Erik Koomen, Karel Allegaert, Manon J.N.L. Benders, Thomas P.C. Dorlo, and Alwin D.R. Huitema have no conflicts of interest to declare that may be relevant to the contents of this article.
Availability of data and material
The raw data are available upon reasonable request by an e-mail to the corresponding author, while blinding for group allocation should be respected until the primary and secondary outcome analyses have been finalized.
Code availability
The codes are available upon reasonable request.
Authors’ contributions
RS, MN, MB, JB, NJ, JN, KvL, EK, and the CRUCIAL study group were responsible for protocol development, study conduct, study registration, study recruitment, and data collection. Population PK modeling was performed by WC, KA, TD, and AH. The draft of this manuscript was written and reviewed by WC, MN, KA, TD, and AH. All authors and contributors have read and commented on the manuscript and have approved the submitted version.
Ethics approval
The CRUCIAL trial (EudraCT 2017-004596-31; ClinicalTrials.gov NCT04217421) was conducted according to the principles of the Helsinki Declaration (2013). The trial was approved by the Medical Research Ethical Committee of UMC Utrecht (reference number NL62772.041.18) and the Dutch national competent authority Central Committee on Research Involving Human Subjects (reference number NL62772.041.18).
Consent for publication
Not applicable.
Consent to participate
Written informed consent was collected from all parents or legal guardians before study participation. Parents were informed about the study by the obstetrician or fetal cardiologist between 24 and 36 weeks of gestation and were asked for written consent before the start of delivery.
Additional information
The members of the CRUCIAL trial consortium are listed in acknowledgements.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chu, WY., Nijman, M., Stegeman, R. et al. Population Pharmacokinetics and Target Attainment of Allopurinol and Oxypurinol Before, During, and After Cardiac Surgery with Cardiopulmonary Bypass in Neonates with Critical Congenital Heart Disease. Clin Pharmacokinet 63, 1205–1220 (2024). https://doi.org/10.1007/s40262-024-01401-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-024-01401-3