Abstract
Background
Laboratory testing is typically required for patients with atopic dermatitis (AD) treated with systemic immunosuppressants. A previous analysis of laboratory outcomes in randomized, double-blinded, placebo-controlled clinical trials of dupilumab in adults with moderate-to-severe AD found no clinically important changes in hematologic, serum chemistry, and urinalysis parameters, supporting the use of dupilumab without routine laboratory monitoring.
Objective
The aim was to assess laboratory results in adolescents with moderate-to-severe AD treated with dupilumab in a phase 3, randomized, double-blind, placebo-controlled trial.
Methods
Adolescents aged ≥ 12 to < 18 years with moderate-to-severe AD were randomized 1:1:1 to subcutaneous dupilumab 200/300 mg every 2 weeks (q2w) (200 mg for patients < 60 kg at baseline; 300 mg for patients ≥ 60 kg at baseline); dupilumab 300 mg every 4 weeks (q4w); or placebo for 16 weeks. Laboratory evaluations included hematology, serum chemistry, and urinalysis parameters.
Results
Of 251 patients enrolled in the study, 250 received treatment and were included in the analysis. 4.7%, 2.4%, and 4.8% of patients receiving placebo, dupilumab 200/300 mg q2w, and dupilumab 300 mg q4w, respectively, had laboratory abnormalities reported as treatment-emergent adverse events, none of which prompted discontinuation of study treatment or study withdrawal. Mean eosinophil counts were elevated at baseline in all treatment groups. Patients in both dupilumab regimens, but not the placebo group, showed mild transient increases in mean eosinophil counts above baseline that returned to near-baseline values by week 16. Mean levels of lactate dehydrogenase trended towards the upper limit of normal at baseline and decreased with treatment; greater decreases were seen in dupilumab-treated patients than placebo-treated patients. There were no meaningful changes in other laboratory parameters, and none of the laboratory abnormalities were clinically significant.
Conclusion
No clinically meaningful changes in laboratory parameters were seen in adolescents, similar to that observed in adults. The findings of this study indicate no routine laboratory monitoring is required in this population prior to or during dupilumab treatment.
Trial Registration
ClinicalTrials.gov: NCT03054428.
Video Abstract
Video abstract: Effect of Dupilumab on Laboratory Parameters in Adolescents with Atopic Dermatitis: Results from a Randomized Placebo-Controlled Phase 3 Clinical Trial (MP4 175137 KB)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Systemic immunosuppressant medications used for atopic dermatitis (AD), such as cyclosporine, methotrexate, and other immunosuppressants, require baseline and serial blood tests. |
Previous analyses from randomized, double-blinded, placebo-controlled trials in adults with moderate-to-severe AD have shown that dupilumab (a monoclonal antibody that specifically blocks the shared receptor component for interleukin-4 and interleukin-13) did not result in any clinically meaningful changes in laboratory parameters for hematology, serum chemistry, and urinalysis. |
An analysis of laboratory parameters from a similar randomized, double-blinded, placebo-controlled trial of dupilumab in adolescents with moderate-to-severe AD identified only clinically insignificant transient eosinophilia and decreases in lactate dehydrogenase, supporting use of dupilumab in this age group without routine laboratory monitoring for hematology, serum chemistry, and urinalysis. |
1 Introduction
The only systemic immunosuppressant treatments approved for use in atopic dermatitis (AD) are corticosteroids in the USA and cyclosporine in Europe, although these and other systemic immunosuppressants have been used off-label for severe AD refractory to topical therapy [1,2,3]. Treatment with these drugs requires baseline and serial laboratory monitoring [4, 5], which increases the treatment burden and may reduce compliance.
Dupilumab is a targeted fully human VelocImmune®-derived [6, 7] monoclonal antibody that specifically blocks the shared receptor component for interleukin (IL)-4 and IL-13, thus inhibiting signaling of both IL-4 and IL-13, which are key and central drivers of type 2 inflammation in multiple diseases [8, 9]. In randomized phase 3 trials in adults and adolescents with moderate-to-severe AD, dupilumab with or without topical corticosteroids improved AD signs, symptoms, and patient quality of life with an acceptable safety profile [10,11,12,13,14,15].
In a previous analysis of laboratory outcomes in clinical trials of dupilumab in adults with moderate-to-severe AD, there were no clinically significant changes in laboratory parameters, supporting the use of dupilumab in this age group without routine laboratory monitoring [16]. We now report laboratory outcomes from a phase 3, randomized, double-blind, placebo-controlled trial in adolescents with moderate-to-severe AD.
2 Methods
2.1 Study Design, Patients, and Treatment
LIBERTY AD ADOL (NCT03054428) was a randomized, placebo-controlled, double-blind, parallel-group, phase 3 trial [13]. The full study design, patient population, efficacy, and safety outcomes have been previously reported [13]. In brief, patients aged ≥ 12 to < 18 years with moderate-to-severe AD whose disease was inadequately controlled with topical treatment or for whom topical treatment was inadvisable were randomized 1:1:1 to 16-week treatment with placebo every 2 weeks (q2w); dupilumab q2w (400-mg loading dose followed by 200 mg q2w for patients with a baseline weight < 60 kg; 600-mg loading dose followed by 300 mg for patients with a baseline weight ≥ 60 kg); or dupilumab 300 mg every 4 weeks (q4w) (600-mg loading dose). Topical corticosteroids and topical calcineurin inhibitors as well as systemic medication for AD were prohibited except for use as rescue medication for intolerable AD symptoms. Specific exclusion criteria related to laboratory abnormalities included platelets ≤ 100 × 109/L, neutrophils < 1.5 × 109/L, creatine phosphokinase > 5 × upper limit of normal (ULN), serum creatinine > 1.5 × ULN, or evidence of liver disease indicated by persistent (confirmed by repeated tests ≥ 2 weeks apart) alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) > 3 × ULN during the screening period [13].
2.2 Ethics
The study was conducted following the ethical principles derived from the Declaration of Helsinki, the International Conference on Harmonisation guidelines, Good Clinical Practice, and local applicable regulatory requirements. Written informed consent was obtained from all patients and the patients’ parents/guardians prior to commencement of any study treatment.
2.3 Laboratory Measurements
Blood and urine were collected for laboratory monitoring at baseline and weeks 4, 8, and 16. Hematology including red blood cell, white blood cell, and platelet parameters; serum chemistry including renal function, liver function, electrolytes, metabolic function, and lipids; and urinalysis assessments (Table 1) were analyzed by a central laboratory (PPD Global Central Labs LLC, Highland Heights, KY, USA).
Investigators were instructed to report laboratory abnormalities as adverse events if one or more of the following occurred:
-
The test result was associated with accompanying symptoms.
-
The test result required additional diagnostic testing or medical/surgical intervention.
-
The test result prompted dose adjustment (outside of those stipulated by the protocol) and/or discontinuation from the study.
-
Management of the event required significant additional concomitant drug treatment or other therapy.
The study drug was to be permanently discontinued in the case of severe laboratory abnormalities, including:
-
Neutrophils ≤ 0.5 × 109/L, platelets ≤ 50 × 109/L
-
ALT and/or AST > 3 × ULN with total bilirubin > 2 × ULN (unless the elevated bilirubin levels were related to confirmed Gilbert’s syndrome)
-
Confirmed AST and/or ALT > 5 × ULN persisting > 2 weeks
If the laboratory abnormality was not suspected to be related to the study drug, treatment was resumed when the abnormality normalized; otherwise, the study drug was to be permanently discontinued if the laboratory abnormality was deemed drug related.
2.4 Statistical Analysis
This analysis used the safety analysis set, which included all randomized patients who received one or more doses of study drug. All statistics are descriptive, using an all-observed values method without any imputation for missing values; all statistics were computed based on the number of available samples at each time point. Analyses included values at baseline and change from baseline by visit as means with standard deviation (SD) or medians (min, max, interquartile range); proportions of patients whose laboratory values shifted between low, normal, or high by visit; and a post hoc analysis of proportions of patients with grades 1, 2, 3, or 4 changes in key variables, i.e., red blood cell parameters, platelets, eosinophils, neutrophils, alkaline phosphatase (ALP), bilirubin, ALT, AST, creatinine, and potassium. Unless otherwise indicated, grades are defined as per Common Terminology Criteria for Adverse Events Version 5.0 [17], where grade 1 is defined as mild, grade 2 as moderate, grade 3 as severe or medically significant but not immediately life-threatening, and grade 4 as potentially life-threatening. For eosinophilia, grades were defined as per the Nordic MPN Study group recommendation, where grade 1 is defined as mild (≥ 0.5 to ≤ 1.5 × 109/L), grade 2 as moderate (> 1.5 to ≤ 5.0 × 109/L), and grade 3 as severe (> 5.0 × 109/L) [18]. A post hoc analysis evaluated the correlation (r) of the maximum increase in eosinophil counts across all visits versus baseline eosinophils, in log-scale, with baseline in log-scale as a covariate; for maximum post-baseline data that are less than or equal to the baseline value, the log (analysis value/baseline) was set to 0. Summary statistics are provided for all variables in the electronic supplementary material (ESM).
3 Results
3.1 Patients
A total of 251 patients were randomized, and 250 were included in the laboratory safety analysis. One patient randomized to the dupilumab q4w group who did not receive study treatment was excluded. Baseline demographics were similar across treatment groups (ESM Table S1), and have been previously reported [13]. A total of 240 patients (95.6%) completed the study. Ten patients had laboratory abnormalities reported as treatment-emergent adverse events (TEAEs) (Table 2), none of which led to treatment or study withdrawal.
3.2 Clinical Laboratory Parameters Reported During the Treatment Period
3.2.1 Hematology
3.2.1.1 Red Blood Cells and Platelets
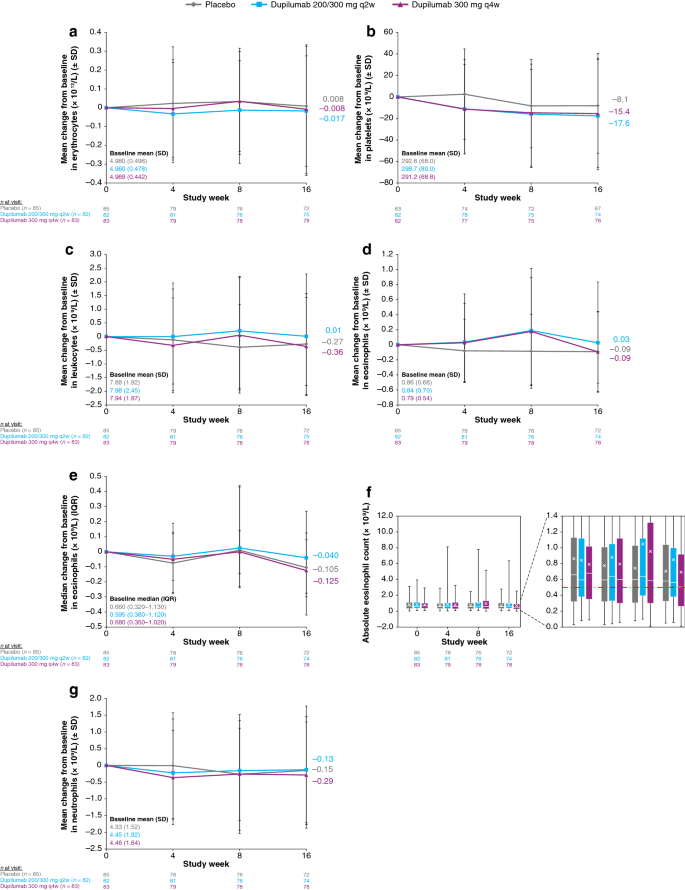
Red blood cell parameters were similar between treatment groups at baseline (ESM Table S2a). There were no clinically meaningful trends or differences between treatment groups in mean or median changes from baseline in red blood cell parameters (Fig. 1a; ESM Table S2a) and there were no clinically meaningful differences between treatment groups in shifts from baseline in any red blood cell parameter (data not shown) nor in the proportion of patients with grades 1–3 anemia (ESM Table S2b) during the treatment period.
Hematology. a Mean change from baseline over time in erythrocytes. b Mean change from baseline over time in platelets. c Mean change from baseline over time in leukocytes. d Mean change from baseline over time in eosinophils. e Median change from baseline over time in eosinophils. f Box plots of absolute eosinophil count over time. Horizontal lines represent medians and X represent means. Error bars show minimum and maximum values. The expanded inset on the right shows a zoom in between 0.0 and 1.4. The dashed red line represents the upper limit of normal. g Mean change from baseline over time in neutrophils. IQR interquartile range, q2w every 2 weeks, q4w every 4 weeks, SD standard deviation
Platelet counts were similar among treatment groups at baseline (ESM Table S2a). Total platelet count decreased slightly over time, with mean changes of − 8.1, − 17.6, and − 15.4 × 109/L for the placebo, dupilumab 200/300-mg q2w, and dupilumab 300-mg q4w groups, respectively (Fig. 1b; ESM Table S2a). A higher proportion of patients in the dupilumab groups than in the placebo group shifted from a high count at baseline to normal by week 16; otherwise, there were no clinically meaningful differences between treatment groups in shifts from baseline in platelet counts during the treatment period (ESM Table S2c). No patients had grade 2 or higher changes from baseline (Table 3). None of these changes were reported as TEAEs, and none were associated with clinical events such as bleeding. Overall, none of the patients had red blood cell or platelet laboratory test abnormalities that led to treatment discontinuation.
3.2.1.2 White Blood Cells
For all white blood cell parameters, counts were similar among treatment groups at baseline (ESM Table S3a). There were no meaningful trends or differences among treatment groups in leukocytes (Fig. 1c; ESM Table S3a).
Mean eosinophil counts were elevated at baseline (0.8–0.9 × 109/L; normal range 0–0.5 × 109/L) in all treatment groups (Fig. 1d; ESM Table S3a). Both dupilumab treatment groups showed transient increases from baseline in mean eosinophil counts, returning to near baseline values by the end of the treatment period, whereas mean eosinophil counts decreased from baseline in the placebo group (Fig. 1d). This pattern was not observed for median change in eosinophil counts (Fig. 1e, f), suggesting that the changes in the means were driven by a minority of patients. These transient increases in eosinophils had no clinical consequences. The proportion of patients with shifts from normal eosinophil counts at baseline to high eosinophil counts at week 16 was similar among treatment groups (25.8%, 22.6%, and 21.4% for placebo, dupilumab q2w, and dupilumab q4w groups, respectively) (ESM Table S3b). No patients had low eosinophil counts (ESM Table S3b). Overall, the proportions of patients with grades 1–3 eosinophilia were generally similar across treatment groups (Table 4). At baseline, the proportions of patients in the placebo, dupilumab q2w, and dupilumab q4w groups with grade 1 eosinophilia were 45.9%, 47.6%, and 54.2%, respectively; proportions with grade 2 eosinophilia were 15.3%, 12.2%, and 10.8%, respectively; and no patients had grade 3 eosinophilia at baseline. At week 4, one patient in the dupilumab q2w group had grade 3 (severe) eosinophilia, and at week 8, one patient each in the dupilumab q2w and q4w groups had grade 3 eosinophilia (Table 4). By week 16, the proportions of patients with grade 1 eosinophilia in the dupilumab groups decreased to levels lower than baseline—proportions were 50.0%, 43.2%, and 38.5% in the placebo, dupilumab q2w, and dupilumab q4w groups, respectively; proportions with grade 2 eosinophilia were 6.9%, 13.5%, and 12.8%, respectively; and one patient in the dupilumab q2w group had grade 3 eosinophilia (Table 4). One patient had a TEAE of eosinophilia, but no eosinophil abnormalities were associated with clinically symptomatic events or treatment discontinuation. Baseline eosinophil levels were inversely correlated with the maximum increase in eosinophil levels during the treatment period; this effect was significant in the placebo group (R = −0.347 [95% confidence interval {CI} − 0.428 to − 0.267]; p < 0.0001) and the dupilumab 200/300-mg q2w group (R = − 0.156 [95% CI − 0.280 to − 0.033]; p = 0.0138), but not in the dupilumab 300-mg q4w group (R = − 0.096 [95% CI − 0.244 to 0.052]; p = 0.2014). Patients with the lowest eosinophil counts at baseline experienced the greatest increase during the treatment period.
Neutrophil counts decreased slightly over time in all groups, with mean changes from baseline of − 0.2, − 0.1, and − 0.3 × 109/L for the placebo, dupilumab 200/300-mg q2w, and dupilumab 300-mg q4w groups, respectively (Fig. 1g; ESM Table S3a). Neutrophils (ESM Table S3b) and other white blood cell parameters (data not shown) showed no clinically meaningful differences between treatment groups in shifts from baseline during the treatment period. Only one to two patients in each treatment group showed transient grades 1–2 changes at any time point; one patient in the dupilumab q4w group showed a transient grade 3 change at week 8; no patients showed grade 4 changes (Table 5).
Overall, no patients had white blood cell laboratory test abnormalities that led to treatment discontinuation, and there were no clinically meaningful trends or differences between treatment groups in other white blood cell parameters during the treatment period (ESM Tables S3a and S3b).
3.2.2 Serum Chemistry
There were no clinically meaningful trends or differences between treatment groups in mean or median changes from baseline or in serum chemistry parameters during the treatment period (Fig. 2a–c; ESM Table S4a–e). Small, clinically irrelevant variations were observed over time in all treatment groups in serum creatinine, serum urate, creatinine phosphokinase, serum potassium, serum bilirubin, serum triglycerides, and total cholesterol (ESM Table S4a–e).
Mean levels of lactate dehydrogenase (LDH) trended towards ULN at baseline and decreased from baseline over time, with more pronounced decreases seen in the dupilumab arms (Fig. 2a; ESM Table S5). A higher proportion of patients in the dupilumab groups shifted from high to normal values in LDH at week 16 (100% and 88.9% for q4w and q2w, respectively) than the placebo group (75%; ESM Table S5). Serum ALP levels were within normal range at baseline and increased from baseline over time in the dupilumab arms, whereas levels decreased in the placebo arm (Fig. 2b; ESM Table S4d). For the other serum chemistry parameters, the proportion of patients who shifted from normal to high in the treatment period was similar between dupilumab and placebo treatment groups.
The proportions of patients showing grades 1–2 changes were small and balanced across treatment groups in most serum chemistry parameters, and no grade 3 or higher abnormalities were reported for any serum chemistry parameters (ESM Table S6a–f). No grade 2 or higher abnormalities were observed for serum ALP, and the proportion of patients with grade 1 abnormalities for ALP at week 16 was low and balanced across the dupilumab and placebo arms. There were four TEAEs of blood creatine phosphokinase increased (Medical Dictionary for Regulatory Activities Preferred Term [MedDRA PT], one patient in the placebo group, one in the dupilumab q2w group, and two in the dupilumab q4w group); two TEAEs of transaminase increase (MedDRA PT; one patient each in the placebo and dupilumab q2w groups); one TEAE of liver function test increase (MedDRA PT; placebo); and one TEAE of hyperuricemia (MedDRA PT; dupilumab q4w) (Table 2). One patient in the placebo group temporarily discontinued treatment due to a transient increase in AST (Table 2); no patients treated with dupilumab had relevant chemistry laboratory abnormalities that led to treatment discontinuation.
3.2.3 Urinalysis
There were no clinically meaningful trends or differences between treatment groups in mean or median changes from baseline in urinalysis parameters during the treatment period (ESM Table S7). A TEAE of mild proteinuria was reported in one patient in the placebo group; this TEAE did not lead to treatment discontinuation (Table 2).
4 Discussion
This analysis found no laboratory outcomes that were of clinical concern and none that indicated a need for routine laboratory monitoring of adolescent patients with moderate-to-severe AD treated with dupilumab. Furthermore, laboratory abnormalities reported as adverse events were uncommon and did not lead to treatment withdrawal or study withdrawal for any patient. In general, laboratory outcomes in this adolescent patient population were similar to those in adults [16].
Overall, there was a trend for a decrease in total platelet count and total neutrophil count over time, similar to what was previously reported in adults [16]. However, the median values in each treatment group were within the normal reference range from baseline to week 16. There were no patients who had grade 2 or higher decreases in platelet and neutrophil counts, and there were no clinically meaningful differences in the number of patients whose counts shifted from normal to low in any of the treatment groups. Neutrophil and platelet counts have been shown to be markers of inflammation in AD, and it is possible that the decrease in neutrophil and platelet count over time was related to reduction in levels of inflammation [19, 20].
Median eosinophil levels were higher at baseline (0.6–0.7 × 109/L [above normal range of 0–0.5 × 109/L]) and other time points in this study than median levels in the previous analysis of studies in adults (0.3–0.4 × 109/L) [16]. Eosinophilia in blood and tissue is common in AD, and levels are associated with AD severity [21,22,23,24,25,26]. A transient increase in mean, but not median, eosinophil count was observed in dupilumab-treated patients, which was not associated with any clinically relevant consequences. The transient increases in mean but not median eosinophil counts most likely reflect a change in a minority of patients. The transient eosinophilia observed is consistent with the hypothesis that dupilumab blocks the migration of eosinophils into tissue by inhibiting IL-4– and IL-13–mediated production of eotaxins but not eosinophil production or egress from bone marrow. This action results in a transient increase in circulating eosinophil counts—a finding consistent with other clinical studies of dupilumab in AD [16], asthma [27, 28], and chronic rhinosinusitis with nasal polyps (CRSwNP) [29]. In those trials, most cases of eosinophilia had no associated clinical consequences, and did not result in treatment interruption or discontinuation.
Approximately 20% of patients had high LDH values at baseline; median values decreased to a greater extent in the dupilumab groups than the placebo group during the treatment period, suggesting normalization of LDH in dupilumab-treated patients compared with placebo, a pattern also reported in the analysis of LDH in adults in dupilumab clinical trials [16]. LDH is considered a marker of tissue damage, and is strongly correlated with disease severity in AD, Staphylococcus aureus colonization, and transepidermal water loss, as well as inversely correlated with stratum corneum hydration [30,31,32,33]. A decrease in LDH values has been shown to correlate with clinical improvements [16], suggesting that this may signify an improvement in skin barrier in the patients in this analysis.
Serum ALP levels were within normal range at baseline, but were increased in dupilumab groups by approximately 9% at week 16. The absence of any other abnormalities in ALT, AST, and bilirubin and the lack of grade 2 or higher increase in ALP levels suggest that the increase in ALP is not due to liver damage. ALP is considered a marker of growth/bone turnover, especially in children and adolescents, in whom ALP is high during periods of increased growth velocity [34]. Interestingly, data from a randomly sampled population of 264,326 children showed that moderate-to-severe AD was associated with significantly shorter stature in children [35], especially when accompanied with sleep disturbances, but not in adolescents or adults, suggesting that impaired growth velocity may not have a long-term impact. In the present study, the relative increases in ALP among patients in the active treatment groups suggest enhanced growth or increased physical activity, possibly related to improved AD.
The lack of clinically relevant laboratory abnormalities differentiates dupilumab, a targeted IL-4/IL-13 inhibitor, from currently used systemic immunosuppressants, which require baseline and serial blood tests, adding to treatment costs and burden on patients [4]. Although none of the commercially available JAK (Janus kinase) inhibitors are approved in pediatric patients, treatment with JAK inhibitors for all currently approved indications in adults (rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and psoriasis; at the time of drafting of this manuscript, JAK inhibitors were not approved for AD in any age group) also requires dose adjustment and serial blood tests [36,37,38].
This randomized, placebo-controlled clinical trial included frequent laboratory assessments using a central laboratory with validated assays. One limitation of this analysis is that patients with laboratory values showing evidence of severe liver and renal impairment and abnormalities in certain other laboratory values (e.g., platelets, neutrophils, creatine phosphokinase, or serum creatinine) during the screening period were excluded from entering the study. Another limitation is the relatively short 16-week period of data collection. However, an open-label extension trial including pediatric patients from all dupilumab AD trials in children and adolescents is ongoing and will provide longer term laboratory safety data for this age group. Finally, investigation of other laboratory parameters such as biomarkers for AD in this population (e.g., IgE, thymus and activation-regulated chemokine) will be addressed in future publications.
5 Conclusions
Treatment with dupilumab did not result in any clinically meaningful change in laboratory parameters in adolescents with moderate-to-severe AD. The laboratory profile in these adolescent patients was generally comparable to that seen in adults [16]. These findings support that dupilumab treatment does not require baseline or follow-up laboratory monitoring for adolescents or adults. Because patient exclusion criteria in this dupilumab trial may have limited the variability of the study population, real‐world evidence, including long‐term data, will provide additional valuable information on the role of laboratory monitoring in dupilumab‐treated patients with AD.
References
Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–49.
Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part II. J Eur Acad Dermatol Venereol. 2012;26:1176–93.
Drucker AM, Eyerick K, de Bruin-Weller M, et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol. 2018;178:768–75.
Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77:623–33.
Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30.
Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111:5147–52.
Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111:5153–8.
Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13:425–37.
Le Floc’h A, Allinne J, Martin J, et al. Dupilumab protects from type 2 inflammation by impacting both systemic and local inflammatory events downstream of IL-4/IL-13 signalling. Allergy. 2020;75:1188–204.
Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–48.
Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–303.
de Bruin-Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to cyclosporine A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178:1083–101.
Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44–56.
Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377–88.
Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85–96.
Wollenberg A, Beck LA, Blauvelt A, et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br J Dermatol. 2020;182:1120–35.
U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE); version 5.0. November 27, 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 20 Dec 2019.
Nordic MPN Study Group. Guidelines for the diagnosis and treatment of eosinophilia. 3rd version. May 2018. https://www.nmpn.org/index.php/guidelines/18-care-program-for-the-diagnosis-and-treatment-of-eosinophilia-3rd-version-may-2018/file. Accessed 20 Aug 2020.
Dogru M, Citli R. The neutrophil-lymphocyte ratio in children with atopic dermatitis: a case-control study. Clin Ter. 2017;168:e262–5.
Jiang Y, Ma W. Assessment of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in atopic dermatitis patients. Med Sci Monitor. 2017;23:1340–6.
Uehara M, Izukura R, Sawai T. Blood eosinophilia in atopic dermatitis. Clin Exp Dermatol. 1990;15:264–6.
Kagi MK, Joller-Jemelka H, Wüthrich B. Correlation of eosinophils, eosinophil cationic protein and soluble interleukin-2 receptor with the clinical activity of atopic dermatitis. Dermatology. 1992;185:88–92.
Kiehl P, Falkenberg K, Vogelbruch M, Kapp A. Tissue eosinophilia in acute and chronic atopic dermatitis: a morphometric approach using quantitative image analysis of immunostaining. Br J Dermatol. 2001;145:720–9.
Liu F-T, Goodarzi H, Chen H-Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allerg Immunol. 2011;41:298–310.
De Grauuw E, Beltraminelli H, Simon HU, et al. Eosinophilia in dermatologic disorders. Immunol Allergy Clin N Am. 2015;35:535–60.
Werfel T, Allam J-P, Biedermann T, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:336–49.
Castro M, Corren J, Pavord I, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486–96.
Rabe K, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–85.
Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–50.
Mukai H, Noguchi T, Kamimura K, Nishioka K, Nishiyama S. Significance of elevated serum LDH (lactate dehydrogenase) activity in atopic dermatitis. J Dermatol. 1990;17:477–81.
Kou K, Alhara M, Matsunaga T, et al. Association of serum interleukin-18 and other biomarkers with disease severity in adults with atopic dermatitis. Arch Dermatol Res. 2012;304:305–12.
Thijs J, Krastev T, Weidinger S, et al. Biomarkers for atopic dermatitis: a systemic review and meta-analysis. Curr Opin Allergy Clin Immunol. 2015;15:453–60.
Simpson EL, Villarreal M, Jepson B, et al. Patients with atopic dermatitis colonized with Staphylococcus aureus have a distinct phenotype and endotype. J Investig Dermatol. 2018;138:2224–33.
Turan S, Topcu B, Gökçe İ, et al. Serum alkaline phosphatase levels in healthy children and evaluation of alkaline phosphatase z-scores in different types of rickets. J Clin Res Pediatr Endocrinol. 2011;3:7–11.
Silverberg JI, Paller AS. Association between eczema and stature in 9 US population-based studies. JAMA Dermatol. 2015;151:401–9.
JAKAFI® (ruxolitinib). Highlights of prescribing information. US FDA 2017. www.accessdata.fda.gov/drugsatfda_docs/label/2017/202192s015lbl.pdf. Accessed 20 Dec 2019.
XELJANZ® (tofacitinib). Highlights of prescribing information. US FDA 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf. Accessed 20 Dec 2019.
OLUMIANT® (baricitinib). Highlights of prescribing information. US FDA 2017. www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924s000lbl.pdf. Accessed 20 Dec 2019.
Acknowledgements
The authors thank the patients and their families for their participation in these studies and their colleagues. Elizabeth Bucknam, BS, Steve Chen, MS, Mbole Ekaney, PhD, Pavel Kovalenko, PhD, Jacqueline Kuritzky, Nelson Rita, BA, George Vlamis, BS, Linda Williams, RPh, Yi Zhang, PhD, and Xiaoping Zhu, PhD, (Regeneron Pharmaceuticals, Inc.), Adriana Mello, PhD, and El-Bdaoui Haddad, PhD (Sanofi), contributed to the study. Medical writing and editorial assistance were provided by Alexandre Houzelle, PhD, and Vicki Schwartz, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. (ClinicalTrials.gov identifier: NCT03054428). The study sponsors participated in the study design; collection, analysis, and interpretation of the data; writing of the report; and the decision to submit the article for publication. Medical writing and editorial assistance were provided by Alexandre Houzelle, PhD, and Vicki Schwartz, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Conflict of interest
Elaine C. Siegfried: Dermavant, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, Inc., Verrica—consultant; GlaxoSmithKline, LEO Pharma, Novan, Pfizer—data and safety monitoring board; Eli Lilly, Janssen, Regeneron Pharmaceuticals, Inc., Stiefel, Verrica—Principal Investigator in clinical trials. Thomas Bieber: AbbVie, Almirall, AnaptysBio, Arena, Asana Biosciences, Astellas, BioVersys, Boehringer Ingelheim, Daichi Sankyo, Davos Biosciences, Dermavant/Roivant Sciences, DS Pharma, Eli Lilly, Evaxion Biotech, FLX Bio, Galapagos/MorphoSys, Galderma, Glenmark, GlaxoSmithKline, Incyte, Kymab, LEO Pharma, L´Oréal, Menlo Therapeutics, Novartis, Pfizer, Pierre Fabre, Sanofi/Regeneron Pharmaceuticals, Inc., UCB—lecturer and/or consultant. Eric L. Simpson: AbbVie, Celgene, Eli Lilly, Galderma, Kyowa Hakko Kirin, LEO Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Tioga—grants; AbbVie, Boehringer Ingelheim, Dermavant, Dermira, Eli Lilly, Forte Bio, Incyte, LEO Pharma, MedImmune, Menlo Therapeutics, Ortho Dermatologics, Pfizer, Pierre Fabre Dermo Cosmetique, Regeneron Pharmaceuticals, Inc., Sanofi, Valeant—personal fees. Amy S. Paller: AbbVie, AnaptysBio, Celgene, Eli Lilly, Galderma, Incyte, KrystalBio, LEO Pharma, Janssen, Novartis, Regeneron Pharmaceuticals, Inc.—investigator; AbbVie, Abeona Therapeutics, Almirall, Amgen, Asana Biosciences, Boehringer Ingelheim, Dermavant, Dermira, Eli Lilly, Exicure, Forte, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, RAPT Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, Sol Gel, UCB—consultant with honorarium. Lisa A. Beck: AbbVie, Allakos, Arena Pharma, AstraZeneca, BenevolentAI, Connect Biopharma, Eli Lilly, Incyte, LEO Pharma, Novartis, Pfizer, Principia Biopharma, RAPT Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi, UCB, Vimalan—consultant. AbbVie, LEO Pharma, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi—investigator; 3M, Gilead, Medtronics, Moderna—owns stocks. Mark Boguniewicz: Regeneron Pharmaceuticals, Inc.—investigator; Regeneron Pharmaceuticals, Inc., Sanofi Genzyme—consultant. Lynda C. Schneider: Regeneron Pharmaceuticals, Inc., DBV Technologies—investigator; Genentech—research support; AbbVie—consultant. Faisal A. Khokhar, Zhen Chen, Paola Mina-Osorio, Ashish Bansal: Regeneron Pharmaceuticals, Inc.—employees and shareholders. Randy Prescilla: Sanofi Genzyme—employee, may hold stock and/or stock options in the company.
Ethics approval
The study was conducted following the ethical principles that derive from the Declaration of Helsinki, International Conference on Harmonization guidelines, Good Clinical Practice, and local applicable regulatory requirements. The trial was overseen by an independent data and safety monitoring board.
Consent to participate
Written informed consent was obtained from all patients and the patients’ parents/guardians prior to commencement of any study treatment.
Consent for publication
Not applicable.
Data sharing statement
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the indication has been approved by a regulatory body, if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
Code availability
Not applicable.
Author contributions
AB contributed to study concept and design. ECS, TB, ELS, ASP, LAB, MB, and LCS acquired data. ZC conducted the statistical analyses on the data. AB drafted the manuscript with the medical writer and created figures. ECS, TB, ELS, ASP, LAB, MB, LCS, FAK, ZC, RP, PM-O, and AB interpreted the data, provided critical feedback on the manuscript, approved the final manuscript for submission, and are accountable for the accuracy and integrity of the manuscript.
Additional information
Digital Features: This article is published with digital features, including a video abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13363634.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Siegfried, E.C., Bieber, T., Simpson, E.L. et al. Effect of Dupilumab on Laboratory Parameters in Adolescents with Atopic Dermatitis: Results from a Randomized, Placebo-Controlled, Phase 3 Clinical Trial. Am J Clin Dermatol 22, 243–255 (2021). https://doi.org/10.1007/s40257-020-00583-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-020-00583-3