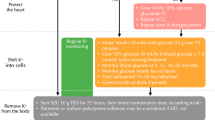
Abstract
This article aims to highlight the dosing issues of direct oral anticoagulants (DOACs) in patients with renal impairment and/or obesity in an attempt to develop solutions employing advanced data-driven techniques. DOACs have become widely accepted by clinicians worldwide because of their superior clinical profiles, more predictable pharmacokinetics, and hence more convenient dosing relative to other anticoagulants. However, the optimal dosing of DOACs in extreme bodyweight patients and patients with renal impairment is difficult to achieve using the conventional dosing approach. The standard dosing approach (fixed-dose) is based on limited data from clinical studies. The existing formulae (models) for determining the appropriate doses for these patient groups leads to suboptimal dosing. This problem of mis-dosing is worsened by the lack of standardized laboratory parameters for monitoring the exposure to DOACs in renal failure and extreme bodyweight patients. Model-informed precision dosing (MIPD) encompasses a range of techniques like machine learning and pharmacometrics modelling, which could uncover key variables and relationships as well as shed more light on the pharmacokinetics and pharmacodynamics of DOACs in patients with extreme bodyweight or renal impairment. Ultimately, this individualized approach—if implemented in clinical practice—could optimise dosing for the DOACs for better safety and efficacy.

Similar content being viewed by others

References
National Institute for Health and Care Excellence NICE. Overview | Anticoagulants, including direct-acting oral anticoagulants (DOACs) [KTT16] | Advice | [Internet]. www.nice.org.uk. 2016. https://www.nice.org.uk/advice/ktt16. Accessed 12 Apr 2022.
Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener H-C, Dubner SJ, et al. The changing landscape for stroke prevention in AF. J Am Coll Cardiol [Internet]. 2017;69(7):777–85.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease. Chest [Internet]. 2016;149(2):315–52.
Chen A, Stecker E, Warden AB. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559.
Mohan A, Wanat MA, Abughosh SM. Medication taking behaviors in patients taking warfarin versus direct oral anticoagulants: a systematic review. Expert Rev Cardiovasc Ther. 2019;17(6):427–34.
Alfirevic A, Downing J, Daras K, Comerford T, Pirmohamed M, Barr B. Has the introduction of direct oral anticoagulants (DOACs) in England increased emergency admissions for bleeding conditions? A longitudinal ecological study. BMJ Open [Internet]. 2020;10(5): e033357.
Schieszer J. Revisiting Guidelines on the Use of Direct Oral Anticoagulants in Obese Patients With Atrial Fibrillation [Internet]. Hematology Advisor. 2019. https://www.hematologyadvisor.com/home/topics/thrombotic-disorders/comparing-clinical-profile-of-doacs-with-warfarin-in-morbidly-obese-patients/. Accessed 12 Apr 2022.
Eschler CM, Antelo A, Funk G-C, Exadaktylos AK, Lindner G. High fluctuation between anticoagulants, frequent off-label dosing, and no difference concerning outcomes: results of a real-life cohort study. Am J Med. 2021;134(3):e165–70.
Sugrue A, Sanborn D, Amin M, Farwati M, Sridhar H, Ahmed A, et al. Inappropriate dosing of direct oral anticoagulants in patients with atrial fibrillation. Am J Cardiol. 2021;144:52–9.
Rymer JA, Webb L, McCall D, Hills MT, Wang TY. Differences in preferences between clinicians and patients for the use and dosing of direct oral anticoagulants for atrial fibrillation. J Am Heart Assoc. 2021;10(11): e020697.
Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. EP Eur. 2021;23:euab065.
White EM, Coons JC. Direct oral anticoagulant use in special populations: elderly, obesity, and renal failure. Curr Cardiol Rep. 2021;23(4):27.
Sarma A, Giugliano RP. Current and developing strategies for monitoring and reversing direct oral anticoagulants in patients with non-valvular atrial fibrillation. Hosp Pract 1995. 2015;43(5):258–67.
Martin KA, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19(8):1874–82.
Pahlmeyer L, Huang J. Monitoring of rivaroxaban levels in patients with class III obesity. Am J Health-Syst Pharm AJHP Off J Am Soc Health-Syst Pharm. 2020;77(13):1013–7.
Ballerie A, Van Nguyen R, Lacut K, Galinat H, Rousseau C, Pontis A, et al. Apixaban and rivaroxaban in obese patients treated for venous thromboembolism: drug levels and clinical outcomes. Thromb Res. 2021;208:39–44.
Nosáľ V, Petrovičová A, Škorňová I, Bolek T, Dluhá J, Stančiaková L, et al. Plasma levels of direct oral anticoagulants in atrial fibrillation patients at the time of embolic stroke: a pilot prospective multicenter study. Eur J Clin Pharmacol. 2022;78(4):557–64.
McCaughan GJB, Favaloro EJ, Pasalic L, Curnow J. Anticoagulation at the extremes of body weight: choices and dosing. Expert Rev Hematol. 2018;11(10):817–28.
Kido K, Lee JC, Hellwig T, Gulseth MP. Use of direct oral anticoagulants in morbidly obese patients. Pharmacotherapy J Human Pharmacol Drug Therapy. 2019;40(1):72–8.
Barras M, Legg A. Drug dosing in obese adults. Aust Prescr [Internet]. 2017;40(5):189–93.
Moll S, Crona DJ, Martin K. Direct oral anticoagulants in extremely obese patients: OK to use? Res Pract Thromb Haemost [Internet]. 2018;3(2):152–5. https://doi.org/10.1002/rth2.12178.
Griggs JJ, Mangu PB, Temin S, Lyman GH. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Oncol Pract. 2012;8(4):e59-61.
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051–65.
Morrish GA, Pai MP, Green B. The effects of obesity on drug pharmacokinetics in humans. Expert Opin Drug Metab Toxicol. 2011;7(6):697–706.
Jackson LR, Schrader P, Thomas L, Steinberg BA, Blanco R, Allen LA, et al. Dosing of direct oral anticoagulants in patients with moderate chronic kidney disease in US clinical practice: results from the outcomes registry for better informed treatment of AF (ORBIT-AF II). Am J Cardiovasc Drugs Drugs Devices Interv. 2021;21(5):553–61.
Ting C, Rhoten M, Dempsey J, Nichols H, Fanikos J, Ruff CT. Evaluation of direct oral anticoagulant prescribing in patients with moderate to severe renal impairment. Clin Appl Thromb. 2021;27:1076029620987900.
Shrestha S, Baser O, Kwong WJ. Effect of Renal function on dosing of non-vitamin K antagonist direct oral anticoagulants among patients with nonvalvular atrial fibrillation. Ann Pharmacother. 2018;52(2):147–53.
Aursulesei V, Costache II. Anticoagulation in chronic kidney disease: from guidelines to clinical practice. Clin Cardiol. 2019;42(8):774–82.
Kcükköylü S, Rump LC. DOAC use in patients with chronic kidney disease. Hamostaseologie. 2017;37(4):286–94.
Lutz J, Jurk K, Schinzel H. Direct oral anticoagulants in patients with chronic kidney disease: patient selection and special considerations. Int J Nephrol Renovasc Dis [Internet]. 2017;10:135–43. https://doi.org/10.2147/IJNRD.S105771.
Yonezawa Y, Horinaka S, Shirakawa C, Kogure Y. Estimated glomerular filtration ratio is a better index than creatinine clearance (Cockcroft–Gault) for predicting the prevalence of atrial fibrillation in the general Japanese population. Hypertens Res. 2018;41(6):451–9.
Zhou L-Y, Yin W-J, Zhao J, Zhang B-K, Hu C, Liu K, et al. A novel creatinine-based equation to estimate glomerular filtration rate in Chinese population with chronic kidney disease: implications for DOACs dosing in atrial fibrillation patients. Front Pharmacol. 2021;12: 615953.
Schwartz JB. Potential effect of substituting estimated glomerular filtration rate for estimated creatinine clearance for dosing of direct oral anticoagulants. J Am Geriatr Soc [Internet]. 2016; 64(10):1996–2002. https://escholarship.org/uc/item/2v67f2f3. Accessed 2019 May 12.
Helldén A, Odar-Cederlöf I, Nilsson G, Sjöviker S, Söderström A, von Euler M, et al. Renal function estimations and dose recommendations for dabigatran, gabapentin and valaciclovir: a data simulation study focused on the elderly. BMJ Open. 2013;3(4): e002686.
Kruger PC, Robinson MA, Xu K, Siegal DM, Eikelboom JW, Bhagirath VC. Assessing renal function in patients receiving DOACs: Cockcroft-Gault versus estimated glomerular filtration rate. Thromb Res. 2017;157:165–6.
Matzke GR, Aronoff GR, Atkinson AJ, Bennett WM, Decker BS, Eckardt K-U, et al. Drug dosing consideration in patients with acute and chronic kidney disease—a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80(11):1122–37.
Pai MP. Estimating the glomerular filtration rate in obese adult patients for drug dosing. Adv Chronic Kidney Dis. 2010;17(5):e53-62.
NHS SPS (Specialist Pharmacy Services). DOACs in renal impairment: practice guide to dosing issues-vs3-Feb 2020 (AW) https://www.sps.nhs.uk/wp-content/uploads/2019/07/DOACs-in-Renal-Impairment-Practice-Guide-to-Dosing-Issues-v3-Feb-2020-AW.pdf.
Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and New CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol CJASN [Internet]. 2010;5(6):1003–9.
Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Internal Med. 2009;150(9):604.
Calsolaro V, Okoye C, Rogani S, Calabrese AM, DellAgnello U, Antognoli R, et al. Different glomerular filtration rate estimating formula for prescribing DOACs in oldest patients: appropriate dosage and bleeding risk Post hoc analysis of a prospective cohort. Aging Clin Exp Res [Internet]. 2021. https://doi.org/10.1007/s40520-021-01986-w.
Parker K, Thachil J. The use of direct oral anticoagulants in chronic kidney disease. Br J Haematol. 2018;183(2):170–84.
Hornum M, Feldt-Rasmussen B. Drug dosing and estimated renal function—any step forward from effersoe? Nephron. 2017;136(4):268–72.
Viceconti M, Pappalardo F, Rodriguez B, Horner M, Bischoff J, Musuamba TF. In silico trials: verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods. 2021;185:120–7.
Tyson RJ, Park CC, Powell JR, Patterson JH, Weiner D, Watkins PB, et al. Precision dosing priority criteria: drug, disease, and patient population variables. Front Pharmacol [Internet]. 2020;11. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85084255688&doi=10.3389%2ffphar.2020.00420&partnerID=40&md5=2b63737b6aa9580b64bb624f279af7bd
Polasek TM, Rostami-Hodjegan A, Yim D-S, Jamei M, Lee H, Kimko H, et al. What does it take to make model-informed precision dosing common practice? Report from the 1st Asian Symposium on Precision Dosing. AAPS J. 2019;21(2):17. https://doi.org/10.1208/s12248-018-0286-6.
Alpaydin E. Introduction to machine learning. Cambridge: The Mit Press; 2014.
(US) Food and Drug Administration (FDA). Guidance for the Use of Bayesian Statistics in Medical Device Clinical Trials. Centre for Biologics Evaluation and Research. Health C for D and R. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-use-bayesian-statistics-medical-device-clinical-trials. Accessed 12 Apr 2022.
Pauly, O. Random Forests for Medical Applications [PhD Thesis, TECHNISCHE UNIVERSITÄT MÜNCHEN]. 2012. http://mediatum.ub.tum.de/doc/1094727/639902.pdf
Hu C, Steingrimsson JA. Personalized risk prediction in clinical oncology research: applications and practical issues using survival trees and random forests. J Biopharm Stat. 2017;28(2):333–49.
Yu W, Liu T, Valdez R, Gwinn M, Khoury MJ. Application of support vector machine modeling for prediction of common diseases: the case of diabetes and pre-diabetes. BMC Med Inform Decis Mak [Internet]. 2010;10:16.
Son Y-J, Kim H-G, Kim E-H, Choi S, Lee S-K. Application of support vector machine for prediction of medication adherence in heart failure patients. Healthc Inform Res [Internet]. 2010;16(4):253–9.
Liu R, Li X, Zhang W, Zhou H-H. Comparison of nine statistical model-based warfarin pharmacogenetic dosing algorithms using the racially diverse international warfarin pharmacogenetic consortium cohort database. PLoS ONE [Internet]. 2015;10(8). https://www.scopus.com/inward/record.uri?eid=2-s2.0-84942900632&doi=10.1371%2fjournal.pone.0135784&partnerID=40&md5=05d125451ad6ee5a988315be173509d5
Bica I, Alaa AM, Lambert C, van der Schaar M. From real-world patient data to individualized treatment effects using machine learning: current and future methods to address underlying challenges. Clin Pharmacol Ther. 2021;109(1):87–100.
Battineni G, Chintalapudi N, Amenta F. Machine learning in medicine: performance calculation of dementia prediction by support vector machines (SVM). Inform Med Unlocked. 2019;16: 100200.
Rahman R, Dhruba SR, Ghosh S, Pal R. Functional random forest with applications in dose-response predictions. Sci Rep. 2019;9(1):1628.
Huang S, Cai N, Pacheco PP, Narrandes S, Wang Y, Xu W. Applications of support vector machine (SVM) learning in cancer genomics. Cancer Genom Proteom. 2018;15(1):41–51.
nQuery Software Team. A Brief Overview of Bayesian Analysis for Biostatisticians. nQuery. 2018. https://blog.statsols.com/a-brief-overview-of-bayesian-analysis-for-biostatisticians
Roychoudhury S. Shaping the Future of Drug Development Practical Model-based Approaches for Phase I Oncology Trial Introduction to Complex Innovative Trial Design Webinar Series [Internet]. 2020. https://www.cytel.com/hubfs/2020%20Webinars/CID%20Series%202020/Introduction%20to%20CID%20Webinar%20Series_PhaseIOnco_Final3.pdf. Accessed 12 Apr 2022.
Bhattacharjee A. Application of Bayesian approach in cancer clinical trial. World J Oncol. 2014;5(3):109–12. https://doi.org/10.14740/wjon842e.
Linden A, Yarnold PR, Nallamothu BK. Using machine learning to model dose–response relationships. J Eval Clin Pract. 2016;22(6):856–63.
Sharabiani A, Bress A, Douzali E, Darabi H. Revisiting Warfarin dosing using machine learning techniques. Comput Math Methods Med. 2015;2015: 560108.
Sharabiani A, Bress A, Galanter W, Nazempour R, Darabi H. A computer-aided system for determining the application range of a warfarin clinical dosing algorithm using support vector machines with a polynomial kernel function. In: IEEE international conference on automation science and engineering [Internet]. 2019. p. 418–23. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85072968500&doi=10.1109%2fCOASE.2019.8842932&partnerID=40&md5=26fb4b61603ecb378e7d7710cf6e2591
Labovitz DL, Shafner L, Gil MR, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke [Internet]. 2017;48(5):1416–9.
Standing JF. Understanding and applying pharmacometric modelling and simulation in clinical practice and research. Br J Clin Pharmacol. 2017;83(2):247–54.
Jha VK, Jairam A, Mahapatra D. Newer oral anticoagulant in chronic kidney disease: what we should know. J Assoc Physicians India. 2019;67:60–5.
Speed V, Green B, Roberts LN, Woolcombe S, Bartoli-Abdou J, Barsam S, et al. Fixed dose rivaroxaban can be used in extremes of bodyweight: a population pharmacokinetic analysis. J Thromb Haemost JTH. 2020;18(9):2296–307.
Willmann S, Zhang L, Frede M, Kubitza D, Mueck W, Schmidt S, et al. Integrated population pharmacokinetic analysis of rivaroxaban across multiple patient populations. CPT Pharmacomet Syst Pharmacol. 2018;7(5):309–20.
Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet [Internet]. 2013;53(1):1–16.
Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59–7939) in healthy subjects. J Clin Pharmacol. 2007;47(2):218–26.
Buller HR, Lensing AWA, Prins MH, Agnelli G, Cohen A, Gallus AS, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein–DVT Dose-Ranging Study. Blood. 2008;112(6):2242–7.
Xu R, Ge W, Jiang Q. Application of physiologically based pharmacokinetic modeling to the prediction of drug-drug and drug-disease interactions for rivaroxaban. Eur J Clin Pharmacol. 2018;74(6):755–65.
Hartmanshenn C, Scherholz M, Androulakis IP. Physiologically-based pharmacokinetic models: approaches for enabling personalized medicine. J Pharmacokinet Pharmacodyn. 2016;43(5):481–504.
Niebecker R, Jönsson S, Karlsson MO, Miller R, Nyberg J, Krekels EHJ, et al. Population pharmacokinetics of edoxaban in patients with symptomatic deep-vein thrombosis and/or pulmonary embolism–the Hokusai-VTE phase 3 study. Br J Clin Pharmacol. 2015;80(6):1374–87.
Yin OQP, Tetsuya K, Miller R. Edoxaban population pharmacokinetics and exposure-response analysis in patients with non-valvular atrial fibrillation. Eur J Clin Pharmacol. 2014;70(11):1339–51.
Xu XS, Moore K, Burton P, Stuyckens K, Mueck W, Rossenu S, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with acute coronary syndromes. Br J Clin Pharmacol. 2012;74(1):86–97.
Rosenbaum S. Basic pharmacokinetics and pharmacodynamics: an integrated textbook and computer simulations. Hoboken: Wiley; 2017.
Barr D, Epps QJ. Direct oral anticoagulants: a review of common medication errors. J Thromb Thrombolysis. 2019;47(1):146–54.
Farhan N, Cristofoletti R, Basu S, Kim S, Lingineni K, Jiang S, et al. Physiologically based pharmacokinetics modeling to investigate formulation factors influencing the generic substitution of dabigatran etexilate. CPT Pharmacomet Syst Pharmacol [Internet]. 2021. https://doi.org/10.1002/psp4.12589.
Toorop MMA, Lijfering WM, Scheres LJJ. The relationship between DOAC levels and clinical outcomes: the measures tell the tale. J Thromb Haemost JTH. 2020;18(12):3163–8.
Ferrat E, Fabre J, Galletout P, Boutin E, Le Breton J, Renard V, et al. Inappropriate direct oral anticoagulant prescriptions in patients with non-valvular atrial fibrillation: cross-sectional analysis of the French CACAO cohort study in primary care. Br J Gen Pract J R Coll Gen Pract. 2021;71(703):e134–9.
Khalil F, Läer S. Physiologically based pharmacokinetic modeling: methodology, applications, and limitations with a focus on its role in pediatric drug development. J Biomed Biotechnol [Internet]. 2011;2011:1–13.
Niederer SA, Aboelkassem Y, Cantwell CD, Corrado C, Coveney S, Cherry EM, et al. Creation and application of virtual patient cohorts of heart models. Philos Trans A Math Phys Eng Sci. 2020;378(2173):20190558.
Madden J, Enoch S, Paini A, Cronin M. A review of in silico tools as alternatives to animal testing: principles, resources and applications. Altern Lab Anim. 2020;48(4):146–72.
Nakamura M, Yamada N, Ito M. Direct oral anticoagulants for the treatment of venous thromboembolism in Japan. J Atheroscler Thromb. 2017;24(6):560–5. https://doi.org/10.5551/jat.RV17005.
Altay O, Ulas M, Ozer M, Genc E (2019) An expert system to predict warfarin dosage in turkish patients depending on genetic and non-genetic factors. In: 2019 7th international symposium on digital forensics and security (ISDFS). https://doi.org/10.1109/isdfs.2019.8757526
Yildirim E, Erol K, Birdane A. Warfarin dose requirement in Turkish patients: the influences of patient characteristics and polymorphisms in CYP2C9, VKORC1 and factor VII. Hippokratia. 2014;18(4):319–27.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Ezekwesiri Michael Nwanosike, Wendy Sunter, Hamid A. Merchant, Barbara R. Conway, Muhammad Ayub Ansari, and Syed Shahzad Hasan declare that they have no potential conflicts.
Availability of data and material
Data sharing does not apply to this article as no datasets were generated or analysed during the current study.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
EMN: conceptualisation, investigation, methodology, project administration, resources, validation, visualisation, and writing the first draft; WS: supervision, validation, reviewing, and editing; HAM: supervision, validation, reviewing, and editing; BRC: supervision, validation, reviewing, and editing; MAA: validation, reviewing, and editing; SSH: conceptualisation, investigation, methodology, project administration, supervision, validation, visualisation, reviewing, and editing.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nwanosike, E.M., Sunter, W., Merchant, H.A. et al. Challenges and Possible Solutions to Direct-Acting Oral Anticoagulants (DOACs) Dosing in Patients with Extreme Bodyweight and Renal Impairment. Am J Cardiovasc Drugs 23, 9–17 (2023). https://doi.org/10.1007/s40256-022-00560-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-022-00560-7