Abstract
Purpose
The aim of this study was to evaluate the population pharmacokinetics (PK) and exposure–response relationship of edoxaban in patients with non-valvular atrial fibrillation (AF).
Methods
Concentration data from 1,134 subjects in 11 clinical studies (eight phase I, one phase II, and two phase III) were used to perform a population PK analysis, including estimation of the bioavailability and quantification of the effects of P-glycoprotein (P-gp) inhibitors as well as renal impairment on edoxaban PK. The potential relationship between edoxaban PK exposure and incidence of bleeding events was explored based on data from 893 AF patients.
Results
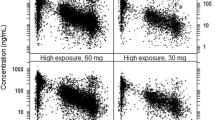
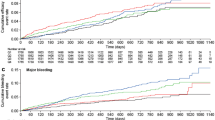
Absolute bioavailability of edoxaban was estimated as 58.3 %. With oral dosing of edoxaban, co-administration of various P-gp inhibitors significantly increased edoxaban bioavailability and decreased volume of distribution (V 2), resulting in a predicted increase of 33–77 % in area under the curve (AUC) and 65–104 % in C max. A much smaller increase was seen in edoxaban concentration at 24 h post-dose (C 24, −24 to 38 %), due to decreased V 2 and shortened elimination half-life. With IV dosing of edoxaban, co-administration of the P-gp inhibitor quinidine decreased both edoxaban clearance (CL) and V 2, resulting in an increase of 32 % in AUC and 66 % in C 24. Creatinine clearance was a significant covariate on renal clearance, whereas age and body weight significantly affected nonrenal clearance. Model-predicted steady state C min was slightly higher, but AUC was comparable for patients who had severe renal impairment and received edoxaban 15 mg once daily (QD) versus patients who had normal renal function or mild renal impairment and received edoxaban 30 mg QD. Exposure–response analysis suggested that edoxaban C min and country/region are significantly associated with the incidence of bleeds.
Conclusions
The model provided reasonable estimation with regard to the absolute bioavailability of edoxaban, the magnitude of change in edoxaban exposure upon co-administration of P-gp inhibitors, and the impact of renal impairment on edoxaban clearance. Analysis results supported a 50 % dose reduction scheme for subjects with severe renal impairment. Further confirmation will be sought by incorporating clinical safety and efficacy information from larger phase III trials.



Similar content being viewed by others
References
Furugohri T, Isobe K, Honda Y, Kamisato-Matsumoto C, Sugiyama N, Nagahara T, Morishima Y, Shibano T (2008) DU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost 6:1542–1549
Raskob G, Cohen AT, Eriksson BI, Puskas D, Shi M, Bocanegra T, Weitz JI (2010) Oral direct factor Xa inhibition with edoxaban for thromboprophylaxis after elective total hip replacement. A randomised double-blind dose-response study. Thromb Haemost 104:642–649
Weitz JI, Connolly SJ, Patel I, Salazar D, Rohatagi S, Mendell J, Kastrissios H, Jin J, Kunitada S (2010) Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost 104:633–641
Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, Hanyok J, Patel I, Shi M, Salazar D, McCabe CH, Braunwald E (2010) Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J 160:635–641
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, ENGAGE AF-TIMI 48 Investigators (2013) Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369:2093–2104
The Hokusai-VTE Investigators (2013) Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 369:1406–1415
Matsushima N, Lee F, Sato T, Weiss D, Mendell J (2013) Bioavailability and safety of the factor Xa inhibitor edoxaban and the effects of quinidine in healthy subjects. Clin Pharmacol Drug Dev 2:358–366
Ogata K, Mendell-Harary J, Tachibana M, Masumoto H, Oguma T, Kojima M, Kunitada S (2010) Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol 50:743–753
Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J (2012) Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos 40:2250–2255
Masumoto H, Yoshigae Y, Watanabe K, Takakusa H, Okazaki O, Izumi T (2010) In vitro metabolism of edoxaban and the enzymes involved in the oxidative metabolism of edoxaban. AAPS J S2:Abstract W4308
Mendell JJL, Ridout G, He L, Chen S (2012) An open-label, phase 1 study to evaluate the effects of hepatic impairment on edoxaban pharmacokinetics. Eur Heart J 33(Suppl 1):Abstract 2024
Graff J, Harder S (2013) Anticoagulant therapy with the oral direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban and the thrombin inhibitor dabigatran etexilate in patients with hepatic impairment. Clin Pharmacokinet 52:243–254
Mikkaichi T, Yoshigae Y, Masumoto H, Imaoka T, Rozehnal V, Fischer T, Okudaira N, Izumi T (2014) Edoxaban transport via P-glycoprotein is a key factor for the drug’s disposition. Drug Metab Dispos 42:520–528
Mendell J, Zahir H, Matsushima N, Noveck R, Lee F, Chen S, Zhang G, Shi M (2013) Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs 13:331–342
Salazar DE, Mendell J, Kastrissios H, Green M, Carrothers TJ, Song S, Patel I, Bocanegra TS, Antman EM, Giugliano RP, Kunitada S, Dornseif B, Shi M, Tachibana M, Zhou S, Rohatagi S (2012) Modelling and simulation of edoxaban exposure and response relationships in patients with atrial fibrillation. Thromb Haemost 107:925–936
Ridout G, de lat Motte S, Sramek P, Johnson L, Ling H, Mendell J, Salazar D (2009) Effect of renal function on edoxaban pharmacokinetics and on population PK/PD model. J Clin Pharmacol 49:1124
Parasrampuria D, Matsushima N, Chen S, Wickremasingha PK, He L, Dishy V, Brown K (2013) Safety, tolerability, and pharmacokinetics of edoxaban in end-stage renal disease subjects undergoing hemodialysis. J Thromb Haemost 11:255–256
Fuji T, Fujita S, Abe Y, Tachibana S, Kawai Y (2013) Evaluation of edoxaban in Japanese patients with severe renal impairment undergoing lower-limb orthopedic surgery. J Thromb Haemost 11:556–557
Koretsune Y, Yamashita T, Yasaka M (2013) Evaluation of edoxaban in patients with atrial fibrillation and severe renal impairment. Eur Heart J 34:95
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Beal SL, Sheiner LB (1998) NONMEM users’ guide. NONMEM Project Group, University of California at San Francisco, San Francisco
Jonsson EN, Karlsson MO (1999) Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Prog Biomed 58:51–64
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NON MEM. Comput Methods Prog Biomed 79:241–257
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Prog Biomed 75:85–94
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic—pharmacodynamic models. I. Models covariate effects. J Pharmacokinet Biopharm 20:511–528
Ananth CV, Kleinbaum DG (1997) Regression models for ordinal responses: a review of methods and applications. Int J Epidemiol 26:1323–1233
Grover A, Benet LZ (2009) Effects of drug transporters on volume of distribution. AAPS J 11:250–261
International Transporter Consortium, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236
Linnet K, Ejsing TB (2008) A review on the impact of P-glycoprotein on the penetration of drugs into the brain. Focus on psychotropic drugs. Eur Neuropsychopharmacol 18:157–169
Lip GY, Larsen TB, Skjøth F, Rasmussen LH (2012) Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol 60:738–746
Goodman SG, Wojdyla DM, Piccini JP, White HD, Paolini JF, Nessel CC, Berkowitz SD, Mahaffey KW, Patel MR, Sherwood MW, Becker RC, Halperin JL, Hacke W, Singer DE, Hankey GJ, Breithardt G, Fox KA, Califf RM, Investigators ROCKETAF (2014) Factors associated with major bleeding events: insights from the ROCKET AF trial (rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol 63:891–900
Hughes M, Lip GY, Guideline Development Group for the NICE national clinical guideline for management of atrial fibrillation in primary and secondary care (2007) Risk factors for anticoagulation-related bleeding complications in patients with atrial fibrillation: a systematic review. Q J Med 100:599–607
Acknowledgments
The authors thank the research staff members who participated in the clinical studies. The authors also thank Dr. Saeheum Song for preparing the analysis dataset and AlphaBioCom for editorial assistance. The analysis was sponsored by Daiichi Sankyo.
Conflict of interest
Ophelia Q. P. Yin, Kimura Tetsuya, and Raymond Miller are employees of Daiichi Sankyo.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 4
(JPEG 212 kb)
Supplementary Fig. 5
(JPEG 204 kb)
Supplementary Fig. 6
(JPEG 178 kb)
Rights and permissions
About this article
Cite this article
Yin, O.Q.P., Tetsuya, K. & Miller, R. Edoxaban population pharmacokinetics and exposure–response analysis in patients with non-valvular atrial fibrillation. Eur J Clin Pharmacol 70, 1339–1351 (2014). https://doi.org/10.1007/s00228-014-1736-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1736-4