Abstract
Aims/Introduction
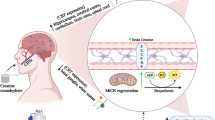
Defective insulin signaling in the brain may disrupt hippocampal neuroplasticity resulting in learning and memory impairments. Thus, this study investigated the effect of aerobic exercise training on cognitive function and synaptic protein markers in diabetic rats.
Materials and methods
Twenty male Wistar rats (200–250 g), were fed on high-fat diet and received a low dose of streptozotocin (35 mg/kg, i.p) to induce type 2 diabetes. Then diabetic animals were randomly divided into sedentary and training groups. The exercise training program was treadmill running at 27 m/min for 60 min/day for 8 weeks. One day after the last training session, Morris Water Maze (MWM) task was performed to evaluate spatial learning and memory. Then, the hippocamp and prefrontal cortex tissues were instantly dissected for immunoblotting assay of BDNF, GSK-3β, p-GSK-3β, P38, p-P38, ERK1/2, p-ERK1/2, heat shock protein-27 (HSP27), SNAP-25, synaptophysin, and PSD-95. Independent t-test analysis and two-way ANOVA was used to determine the differences under significance level of 0.05 using the 26th version of IBM SPSS statistical software.
Results
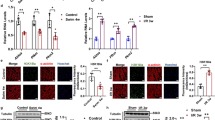
The results showed that aerobic exercise improved memory as assessed in the MWM task. Moreover, aerobic exercise up-regulated HSP27 and BDNF protein levels in the prefrontal cortex, and hippocampus coincided with robust elevations in SNAP25 and PSD-95 levels. Moreover, exercise reduced phosphorylated P38, while increased p-ERK1/2 and p-GSK-3β (p).
Conclusion
Our findings suggest that aerobic exercise may debilitate the harmful effects of diabetes on the cognitive function possibly through enhancing synaptic protein markers.




Similar content being viewed by others
Data availability
Data are all contained within the article.
References
International Diabetes Federation. IDF Diabetes Atlas 9th edn. Belgium: Brussels; 2019.
Carvalho C, et al. Alzheimer’s disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2015;1852(8):1665–75.
Piralaiy E, et al. Cardiac autonomic modulation in response to three types of Exercise in patients with type 2 Diabetic Neuropathy. J Diabetes Metab Diosord. 2021;20(2):1469–78.
Ferrario CR, Reagan LP. Insulin-mediated synaptic plasticity in the CNS: anatomical, functional and temporal contexts. Neuropharmacology. 2018;136:182–91.
Fadel JR, Reagan LP. Stop signs in hippocampal insulin signaling: the role of insulin resistance in structural, functional and behavioral deficits. Curr Opin Behav Sci. 2016;9:47–54.
van der Zee EA. Synapses, spines and kinases in mammalian learning and memory, and the impact of aging. Neurosci Biobehav Rev. 2015;50:77–85.
Hooper PL, et al. The central role of heat shock factor 1 in synaptic fidelity and memory consolidation. Cell Stress Chaperones. 2016;21(5):745–53.
Robinson JL, et al. Perforant path synaptic loss correlates with cognitive impairment and Alzheimer’s Disease in the oldest-old. Brain. 2014;137(9):2578–87.
Kujach S, et al. Acute sprint interval exercise increases both cognitive functions and peripheral neurotrophic factors in humans: the possible involvement of lactate. Front Neurosci. 2020;13: 1455.
Mahalakshmi B, et al. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int J Mol Sci. 2020;21(16): 5895.
Karimi SA, et al. Effects of regular Exercise on Diabetes-Induced memory deficits and biochemical parameters in male rats. J Mol Neurosci. 2021;71(5):1023–30.
Ramos-Miguel A, et al. Exercise prevents downregulation of hippocampal presynaptic proteins following olanzapine-elicited metabolic dysregulation in rats: distinct roles of inhibitory and excitatory terminals. Neurosci. 2015;301:298–311.
Diba R, et al. Protective effects of troxerutin on maternal high-fat diet-induced impairments of spatial memory and apelin in the male offspring. Iran J Basic Med Sci. 2018;21(7):682.
Khani M, et al. Effect of thyme extract supplementation on lipid peroxidation, antioxidant capacity, PGC-1α content and endurance exercise performance in rats. J Int Soc Sports Nutr. 2017;14(1):1–8.
Shima T, et al. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 Diabetes. Diabetologia. 2017;60(3):597–606.
Farajdokht F, et al. Inhibition of PTEN protects PC12 cells against oxygen-glucose deprivation induced cell death through mitoprotection. Brain Res. 2018;1692:100–9.
de Reyes L, Casas-Tintó S. Neural functions of small heat shock proteins. Neural Regen Res. 2022;17(3):512.
Jakhotia S, et al. Circulating levels of Hsp27 in microvascular Complications of Diabetes: prospects as a biomarker of diabetic Nephropathy. J Diabetes Compl. 2018;32(2):221–5.
Pourhamidi K, et al. Heat shock protein 27 is associated with better nerve function and fewer signs of neuropathy. Diabetologia. 2011;54(12):3143–9.
Tóth ME, et al. Overexpression of Hsp27 ameliorates symptoms of Alzheimer’s Disease in APP/PS1 mice. Cell Stress Chaperones. 2013;18(6):759–71.
Henstridge DC, Febbraio MA, Hargreaves M. Heat shock proteins and exercise adaptations. Our knowledge thus far and the road still ahead. J Appl Physiol. 2016;120(6):683–91.
Kim OY, Song J. The importance of BDNF and RAGE in diabetes-induced Dementia. Pharmacol Res. 2020;160: 105083.
He S, et al. Burnout and cognitive impairment: associated with serum BDNF in a Chinese Han population. Psychoneuroendocrinology. 2017;77:236–43.
Kim T-W, et al. High-intensity exercise improves cognitive function and hippocampal brain-derived neurotrophic factor expression in obese mice maintained on high-fat diet. J Exerc Rehabilitation. 2020;16(2):124.
Andrews SC, et al. Intensity matters: high-intensity interval exercise enhances motor cortex plasticity more than moderate exercise. Cereb Cortex. 2020;30(1):101–12.
Jamali A, Shahrbanian S, Tayebi SM. The effects of exercise training on the brain-derived neurotrophic factor (BDNF) in the patients with type 2 Diabetes: a systematic review of the Randomized controlled trials. J Diabetes Metabolic Disorders. 2020;19(1):633–43.
Albert-Gascó H, et al. MAP/ERK signaling in developing cognitive and emotional function and its effect on pathological and neurodegenerative processes. Int J Mol Sci. 2020;21(12): 4471.
Medina JH, Viola H. ERK1/2: a key cellular component for the formation, retrieval, reconsolidation and persistence of memory. Front Mol Neurosci. 2018;11: 361.
Rutigliano G, et al. An isoform-selective p38αMAPK inhibitor rescues early entorhinal cortex dysfunctions in a mouse model of Alzheimer’s Disease. Neurobiol Aging. 2018;70:86–91.
Dai H-l, et al. 38 MAPK inhibition improves synaptic plasticity and memory in angiotensin II-dependent hypertensive mice. Sci Rep. 2016;6: 27600.
Munoz L, et al. A novel p38α MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer’s Disease mouse model. J Neuroinflammation. 2007;4(1): 21.
Song C, et al. AMPK/p38/Nrf2 activation as a protective feedback to restrain oxidative stress and inflammation in microglia stimulated with sodium fluoride. Chemosphere. 2020;244: 125495.
Gupta V, et al. Brain derived neurotrophic factor is involved in the regulation of glycogen synthase kinase 3β (GSK3β) signalling. Biochem Biophys Res Commun. 2014;454(3):381–6.
Ochs S, et al. Loss of neuronal GSK3β reduces dendritic spine stability and attenuates excitatory synaptic transmission via β-catenin. Mol Psychiatry. 2015;20(4):482.
Irfan M, et al. SNAP-25 isoforms differentially regulate synaptic transmission and long-term synaptic plasticity at central synapses. Sci Rep. 2019;9(1):1–14.
Nikukheslat SD, Karimi P, Sadri I. Effect of aerobic training on Synaptic Integrity Proteins in Hippocampus and Prefrontal Cortex of type II Diabetic rats. Med J Tabriz Univ Med Sci. 2020;42(2):160–7.
Hu S, et al. Exercise can increase small heat shock proteins (sHSP) and pre- and post-synaptic proteins in the hippocampus. Brain Res. 2009;1249:191–201.
Liu Y-F, et al. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90(1):81–9.
Perdigão C, et al. Intracellular trafficking mechanisms of synaptic dysfunction in Alzheimer’s Disease. Front Cell Neurosci. 2020;14: 72.
Ye Q, et al. Ferrostatin-1 mitigates cognitive impairment of epileptic rats by inhibiting P38 MAPK activation. Epilepsy Behav. 2020;103:106670.
Yaribeygi H, et al. Neuromodulatory effects of anti-diabetes medications: a mechanistic review. Pharmacol Res. 2020;152: 104611.
Fernandes J, et al. Aerobic exercise attenuates inhibitory avoidance memory deficit induced by paradoxical sleep deprivation in rats. Brain Res. 2013;1529:66–73.
Ferreira AF, et al. Short-term, moderate exercise is capable of inducing structural, BDNF-independent hippocampal plasticity. Brain Res. 2011;1425:111–22.
Cassilhas R, et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neurosci. 2012;202:309–17.
Ferreira AF, et al. Moderate exercise changes synaptic and cytoskeletal proteins in motor regions of the rat brain. Brain Res. 2010;1361:31–42.
Nie J, Yang X. Modulation of synaptic plasticity by exercise training as a basis for ischemic Stroke rehabilitation. Cell Mol Neurobiol. 2017;37(1):5–16.
Dore K, et al. PSD-95 protects synapses from β-amyloid. Cell Rep. 2021;35(9): 109194.
Wang R, et al. Quercetin attenuates diabetic neuropathic pain by inhibiting mTOR/p70S6K pathway-mediated changes of synaptic morphology and synaptic protein levels in spinal dorsal horn of db/db mice. Eur J Pharmacol. 2020;882: 173266.
Arnold SE, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87.
Grillo C, et al. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136(2):477–86.
Kratschke MM. Investigating PSD-95 turnover at the synapse using the HaloTag technology. In: Centre for clinical Brain sciences. Edinburgh: University of Edinburgh; 2018. p. 322.
Jaworski T, Banach-Kasper E, Gralec K. GSK-3β at the intersection of neuronal plasticity and neurodegeneration. Neural Plast 2019; 2019:14.
Acknowledgements
We are grateful to the Neurosciences Research Center (NSRC), Tabriz University of Medical Sciences, especially Dr. Farhoudi for their laboratory equipment, exercise facilities, and technical support.
Funding
The fund for this study was provided by Dr. Iraj Sadri. This fund was used to purchase the necessary supplies. Authors also declare that no funds, grants, or other support were received during the preparation of this manuscript from other organizations or institutes.
Author information
Authors and Affiliations
Contributions
I.S. designed and performed research, analyzed data, and wrote the manuscript. S.D.N. contributed new reagents, and analytical tools and analyzed data and reviewed and edited the manuscript. P.K. analyzed data, and contributed to the discussion and reviewed and edited the manuscript. M.K. designed research, analyzed data, contributed to the discussion, and reviewed and edited the manuscript. S.N. Cooperated in running exercise protocol and data extraction, analyzed data, and wrote the manuscript I.S. is the sponsor of this work and has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the research ethics committee (REC) of Tabriz University of Medical Sciences (TUOMS) (IR.TBZMED.REC.1395.1225).
Consent to participate
Not applicable.
Consent for publication
All the participants gave their consent for the publication of identifiable details, which can include photograph(s) and/or videos and/or case history and/or details within the text (“Material”) to be published in the Journal of diabetes and metabolic disorders and the present article.
Conflicts of interest/Competing interests
There are no competing conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadri, I., Nikookheslat, S.D., Karimi, P. et al. Aerobic exercise training improves memory function through modulation of brain-derived neurotrophic factor and synaptic proteins in the hippocampus and prefrontal cortex of type 2 diabetic rats. J Diabetes Metab Disord (2023). https://doi.org/10.1007/s40200-023-01360-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40200-023-01360-9