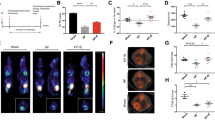
Abstract
Exercise improves cardiac function and metabolism. Although long-term exercise leads to circulating and micro-environmental metabolic changes, the effect of exercise on protein post-translational lactylation modifications as well as its functional relevance is unclear. Here, we report that lactate can regulate cardiomyocyte changes by improving protein lactylation levels and elevating intracellular N6-methyladenosine RNA-binding protein YTHDF2. The intrinsic disorder region of YTHDF2 but not the RNA m6A-binding activity is indispensable for its regulatory function in influencing cardiomyocyte cell size changes and oxygen glucose deprivation/re-oxygenation (OGD/R)-stimulated apoptosis via upregulating Ras GTPase-activating protein-binding protein 1 (G3BP1). Downregulation of YTHDF2 is required for exercise-induced physiological cardiac hypertrophy. Moreover, myocardial YTHDF2 inhibition alleviated ischemia/reperfusion-induced acute injury and pathological remodeling. Our results here link lactate and lactylation modifications with RNA m6A reader YTHDF2 and highlight the physiological importance of this innovative post-transcriptional intrinsic regulation mechanism of cardiomyocyte responses to exercise. Decreasing lactylation or inhibiting YTHDF2/G3BP1 might represent a promising therapeutic strategy for cardiac diseases.








Similar content being viewed by others
Data availability
All data used in this study are available from the authors on reasonable request.
References
Bei Y, Huang Z, Feng X, Li L, Wei M, Zhu Y, Liu S, Chen C, Yin M, Jiang H, Xiao J (2022) Lymphangiogenesis contributes to exercise-induced physiological cardiac growth. J Sport Health Sci 11:466–478. https://doi.org/10.1016/j.jshs.2022.02.005
Bei Y, Pan LL, Zhou Q, Zhao C, Xie Y, Wu C, Meng X, Gu H, Xu J, Zhou L, Sluijter JPG, Das S, Agerberth B, Sun J, Xiao J (2019) Cathelicidin-related antimicrobial peptide protects against myocardial ischemia/reperfusion injury. BMC Med 17:42. https://doi.org/10.1186/s12916-019-1268-y
Bei Y, Wang L, Ding R, Che L, Fan Z, Gao W, Liang Q, Lin S, Liu S, Lu X, Shen Y, Wu G, Yang J, Zhang G, Zhao W, Guo L, Xiao J (2021) Animal exercise studies in cardiovascular research: current knowledge and optimal design-A position paper of the Committee on Cardiac Rehabilitation, Chinese Medical Doctors’ Association. J Sport Health Sci 10:660–674. https://doi.org/10.1016/j.jshs.2021.08.002
Bernardo BC, Ooi JYY, Weeks KL, Patterson NL, McMullen JR (2018) Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: current knowledge and emerging concepts. Physiol Rev 98:419–475. https://doi.org/10.1152/physrev.00043.2016
Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM (2010) C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143:1072–1083. https://doi.org/10.1016/j.cell.2010.11.036
Chang K, Marran K, Valentine A, Hannon GJ (2013) Creating an miR30-based shRNA vector. Cold Spring Harb Protoc 2013:631–635. https://doi.org/10.1101/pdb.prot075853
Chen S, Cao X, Zhang J, Wu W, Zhang B, Zhao F (2022) circVAMP3 drives CAPRIN1 phase separation and inhibits hepatocellular carcinoma by suppressing c-Myc translation. Adv Sci (Weinh). https://doi.org/10.1002/advs.202103817
Chen X, Zhou X, Wang X (2022) m(6)A binding protein YTHDF2 in cancer. Exp Hematol Oncol 11:21. https://doi.org/10.1186/s40164-022-00269-y
Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, Febbraio MA, Galis ZS, Gao Y, Haus JM, Lanza IR, Lavie CJ, Lee CH, Lucia A, Moro C, Pandey A, Robbins JM, Stanford KI, Thackray AE, Villeda S, Watt MJ, Xia A, Zierath JR, Goodpaster BH, Snyder MP (2022) Exerkines in health, resilience and disease. Nat Rev Endocrinol 18:273–289. https://doi.org/10.1038/s41574-022-00641-2
Dai X, Lv X, Thompson EW, Ostrikov KK (2022) Histone lactylation: epigenetic mark of glycolytic switch. Trends Genet 38:124–127. https://doi.org/10.1016/j.tig.2021.09.009
Deng X, Qing Y, Horne D, Huang H, Chen J (2023) The roles and implications of RNA m(6)A modification in cancer. Nat Rev Clin Oncol 20:507–526. https://doi.org/10.1038/s41571-023-00774-x
Dou X, Huang L, Xiao Y, Liu C, Li Y, Zhang X, Yu L, Zhao R, Yang L, Chen C, Yu X, Gao B, Qi M, Gao Y, Shen B, Sun S, He C, Liu J (2023) METTL14 is a chromatin regulator independent of its RNA N6-methyladenosine methyltransferase activity. Protein Cell 14:683–697. https://doi.org/10.1093/procel/pwad009
Fan M, Yang K, Wang X, Chen L, Gill PS, Ha T, Liu L, Lewis NH, Williams DL, Li C (2023) Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci Adv 9:eadc9465. https://doi.org/10.1126/sciadv.adc9465
Fang R, Chen X, Zhang S, Shi H, Ye Y, Shi H, Zou Z, Li P, Guo Q, Ma L, He C, Huang S (2021) EGFR/SRC/ERK-stabilized YTHDF2 promotes cholesterol dysregulation and invasive growth of glioblastoma. Nat Commun 12:177. https://doi.org/10.1038/s41467-020-20379-7
Flamand MN, Tegowski M, Meyer KD (2023) The proteins of mRNA modification: writers, readers, and erasers. Annu Rev Biochem 92:145–173. https://doi.org/10.1146/annurev-biochem-052521-035330
Franzago M, Pilenzi L, Di Rado S, Vitacolonna E, Stuppia L (2022) The epigenetic aging, obesity, and lifestyle. Front Cell Dev Biol 10:985274. https://doi.org/10.3389/fcell.2022.985274
Fu Y, Zhuang X (2020) m(6)A-binding YTHDF proteins promote stress granule formation. Nat Chem Biol 16:955–963. https://doi.org/10.1038/s41589-020-0524-y
Gaffney DO, Jennings EQ, Anderson CC, Marentette JO, Shi T, Schou Oxvig AM, Streeter MD, Johannsen M, Spiegel DA, Chapman E, Roede JR, Galligan JJ (2020) Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem Biol 27(206–213):e206. https://doi.org/10.1016/j.chembiol.2019.11.005
Gao R, Wang L, Bei Y, Wu X, Wang J, Zhou Q, Tao L, Das S, Li X, Xiao J (2021) Long noncoding RNA cardiac physiological hypertrophy-associated regulator induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury. Circulation 144:303–317. https://doi.org/10.1161/CIRCULATIONAHA.120.050446
Gatsiou A, Stellos K (2023) RNA modifications in cardiovascular health and disease. Nat Rev Cardiol 20:325–346. https://doi.org/10.1038/s41569-022-00804-8
Ge Y, Jin J, Li J, Ye M, Jin X (2022) The roles of G3BP1 in human diseases (review). Gene 821:146294. https://doi.org/10.1016/j.gene.2022.146294
Gibb AA, Hill BG (2018) Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res 123:107–128. https://doi.org/10.1161/CIRCRESAHA.118.312017
Gilbert WV, Nachtergaele S (2023) mRNA regulation by RNA modifications. Annu Rev Biochem 92:175–198. https://doi.org/10.1146/annurev-biochem-052521-035949
Glancy B, Kane DA, Kavazis AN, Goodwin ML, Willis WT, Gladden LB (2021) Mitochondrial lactate metabolism: history and implications for exercise and disease. J Physiol 599:863–888. https://doi.org/10.1113/JP278930
Goodwin GW, Taegtmeyer H (2000) Improved energy homeostasis of the heart in the metabolic state of exercise. Am J Physiol Heart Circ Physiol 279:H1490-1501. https://doi.org/10.1152/ajpheart.2000.279.4.H1490
He M, Yang Z, Abdellatif M, Sayed D (2015) GTPase activating protein (Sh3 Domain) binding protein 1 regulates the processing of microRNA-1 during cardiac hypertrophy. PLoS ONE 10:e0145112. https://doi.org/10.1371/journal.pone.0145112
Hesser CR, Karijolich J, Dominissini D, He C, Glaunsinger BA (2018) N6-methyladenosine modification and the YTHDF2 reader protein play cell type specific roles in lytic viral gene expression during Kaposi’s sarcoma-associated herpesvirus infection. PLoS Pathog 14:e1006995. https://doi.org/10.1371/journal.ppat.1006995
Heusch G (2020) Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17:773–789. https://doi.org/10.1038/s41569-020-0403-y
Heusch G (2024) Myocardial ischemia/reperfusion: Translational pathophysiology of ischemic heart disease. Med 5:10–31. https://doi.org/10.1016/j.medj.2023.12.007
Hou G, Zhao X, Li L, Yang Q, Liu X, Huang C, Lu R, Chen R, Wang Y, Jiang B, Yu J (2021) SUMOylation of YTHDF2 promotes mRNA degradation and cancer progression by increasing its binding affinity with m6A-modified mRNAs. Nucleic Acids Res 49:2859–2877. https://doi.org/10.1093/nar/gkab065
Hou J, Zhang H, Liu J, Zhao Z, Wang J, Lu Z, Hu B, Zhou J, Zhao Z, Feng M, Zhang H, Shen B, Huang X, Sun B, Smyth MJ, He C, Xia Q (2019) YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma. Mol Cancer 18:163. https://doi.org/10.1186/s12943-019-1082-3
Izzo LT, Wellen KE (2019) Histone lactylation links metabolism and gene regulation. Nature 574:492–493. https://doi.org/10.1038/d41586-019-03122-1
Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, Yang C, Chen Y (2021) The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther 6:74. https://doi.org/10.1038/s41392-020-00450-x
Kanatsu-Shinohara M, Shinohara T (2013) Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol 29:163–187. https://doi.org/10.1146/annurev-cellbio-101512-122353
Kleinbongard P, Lieder H, Skyschally A, Heusch G (2023) No sex-related differences in infarct size, no-reflow, and protection by ischaemic pre-conditioning in Gottingen minipigs. Cardiovasc Res 119:561–570. https://doi.org/10.1093/cvr/cvac062
Kmietczyk V, Oelschlager J, Gupta P, Varma E, Hartl S, Furkel J, Konstandin M, Marx A, Loewenthal Z, Kamuf-Schenk V, Jurgensen L, Stroh C, Gorska A, Martin-Garrido A, Heineke J, Jakobi T, Frey N, Volkers M (2023) Ythdf2 regulates cardiac remodeling through its mRNA target transcripts. J Mol Cell Cardiol 181:57–66. https://doi.org/10.1016/j.yjmcc.2023.06.001
Koziol MJ, Bradshaw CR, Allen GE, Costa ASH, Frezza C, Gurdon JB (2016) Identification of methylated deoxyadenosines in vertebrates reveals diversity in DNA modifications. Nat Struct Mol Biol 23:24–30. https://doi.org/10.1038/nsmb.3145
Kumari R, Ranjan P, Suleiman ZG, Goswami SK, Li J, Prasad R, Verma SK (2022) mRNA modifications in cardiovascular biology and disease: with a focus on m6A modification. Cardiovasc Res 118:1680–1692. https://doi.org/10.1093/cvr/cvab160
Lee JM, Hammaren HM, Savitski MM, Baek SH (2023) Control of protein stability by post-translational modifications. Nat Commun 14:201. https://doi.org/10.1038/s41467-023-35795-8
Li F, Zhao D, Wu J, Shi Y (2014) Structure of the YTH domain of human YTHDF2 in complex with an m(6)A mononucleotide reveals an aromatic cage for m(6)A recognition. Cell Res 24:1490–1492. https://doi.org/10.1038/cr.2014.153
Li H, Trager LE, Liu X, Hastings MH, Xiao C, Guerra J, To S, Li G, Yeri A, Rodosthenous R, Silverman MG, Das S, Ambardekar AV, Bristow MR, Gonzalez-Rosa JM, Rosenzweig A (2022) lncExACT1 and dchs2 regulate physiological and pathological cardiac growth. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.121.056850
Li L, Krasnykov K, Homolka D, Gos P, Mendel M, Fish RJ, Pandey RR, Pillai RS (2022) The XRN1-regulated RNA helicase activity of YTHDC2 ensures mouse fertility independently of m(6)A recognition. Mol Cell 82(1678–1690):e1612. https://doi.org/10.1016/j.molcel.2022.02.034
Lin H, Zhu Y, Zheng C, Hu D, Ma S, Chen L, Wang Q, Chen Z, Xie J, Yan Y, Huang X, Liao W, Kitakaze M, Bin J, Liao Y (2021) Antihypertrophic memory after regression of exercise-induced physiological myocardial hypertrophy is mediated by the long noncoding RNA Mhrt779. Circulation 143:2277–2292. https://doi.org/10.1161/CIRCULATIONAHA.120.047000
Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Bostrom P, Che L, Zhang C, Spiegelman BM, Rosenzweig A (2015) miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21:584–595. https://doi.org/10.1016/j.cmet.2015.02.014
Markmiller S, Soltanieh S, Server KL, Mak R, Jin W, Fang MY, Luo EC, Krach F, Yang D, Sen A, Fulzele A, Wozniak JM, Gonzalez DJ, Kankel MW, Gao FB, Bennett EJ, Lecuyer E, Yeo GW (2018) Context-dependent and disease-specific diversity in protein interactions within stress granules. Cell 172(590–604):e513. https://doi.org/10.1016/j.cell.2017.12.032
McShane E, Selbach M (2022) physiological functions of intracellular protein degradation. Annu Rev Cell Dev Biol 38:241–262. https://doi.org/10.1146/annurev-cellbio-120420-091943
Moreira JBN, Wohlwend M, Wisloff U (2020) Exercise and cardiac health: physiological and molecular insights. Nat Metab 2:829–839. https://doi.org/10.1038/s42255-020-0262-1
Moreno-Yruela C, Zhang D, Wei W, Baek M, Liu W, Gao J, Dankova D, Nielsen AL, Bolding JE, Yang L, Jameson ST, Wong J, Olsen CA, Zhao Y (2022) Class I histone deacetylases (HDAC1–3) are histone lysine delactylases. Sci Adv 8:eabi6696. https://doi.org/10.1126/sciadv.abi6696
Mu M, Li X, Dong L, Wang J, Cai Q, Hu Y, Wang D, Zhao P, Zhang L, Zhang D, Cheng S, Tan L, Wu F, Shi YG, Xu W, Shi Y, Shen H (2023) METTL14 regulates chromatin bivalent domains in mouse embryonic stem cells. Cell Rep 42:112650. https://doi.org/10.1016/j.celrep.2023.112650
Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15:387–407. https://doi.org/10.1038/s41569-018-0007-y
O’Brown ZK, Boulias K, Wang J, Wang SY, O’Brown NM, Hao Z, Shibuya H, Fady PE, Shi Y, He C, Megason SG, Liu T, Greer EL (2019) Sources of artifact in measurements of 6mA and 4mC abundance in eukaryotic genomic DNA. BMC Genom 20:445. https://doi.org/10.1186/s12864-019-5754-6
Olivares AO, Baker TA, Sauer RT (2018) Mechanical protein unfolding and degradation. Annu Rev Physiol 80:413–429. https://doi.org/10.1146/annurev-physiol-021317-121303
Paris J, Morgan M, Campos J, Spencer GJ, Shmakova A, Ivanova I, Mapperley C, Lawson H, Wotherspoon DA, Sepulveda C, Vukovic M, Allen L, Sarapuu A, Tavosanis A, Guitart AV, Villacreces A, Much C, Choe J, Azar A, van de Lagemaat LN, Vernimmen D, Nehme A, Mazurier F, Somervaille TCP, Gregory RI, O’Carroll D, Kranc KR (2019) Targeting the RNA m(6)A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell 25(137–148):e136. https://doi.org/10.1016/j.stem.2019.03.021
Rabinowitz JD, Enerback S (2020) Lactate: the ugly duckling of energy metabolism. Nat Metab 2:566–571. https://doi.org/10.1038/s42255-020-0243-4
Ratel D, Ravanat JL, Charles MP, Platet N, Breuillaud L, Lunardi J, Berger F, Wion D (2006) Undetectable levels of N6-methyl adenine in mouse DNA: cloning and analysis of PRED28, a gene coding for a putative mammalian DNA adenine methyltransferase. FEBS Lett 580:3179–3184. https://doi.org/10.1016/j.febslet.2006.04.074
Rho H, Terry AR, Chronis C, Hay N (2023) Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab 35(1406–1423):e1408. https://doi.org/10.1016/j.cmet.2023.06.013
Ries RJ, Zaccara S, Klein P, Olarerin-George A, Namkoong S, Pickering BF, Patil DP, Kwak H, Lee JH, Jaffrey SR (2019) m(6)A enhances the phase separation potential of mRNA. Nature 571:424–428. https://doi.org/10.1038/s41586-019-1374-1
Schiffers S, Ebert C, Rahimoff R, Kosmatchev O, Steinbacher J, Bohne AV, Spada F, Michalakis S, Nickelsen J, Muller M, Carell T (2017) Quantitative LC-MS provides no evidence for m(6) dA or m(4) dC in the genome of mouse embryonic stem cells and tissues. Angew Chem Int Ed Engl 56:11268–11271. https://doi.org/10.1002/anie.201700424
Su R, Dong L, Li Y, Gao M, He PC, Liu W, Wei J, Zhao Z, Gao L, Han L, Deng X, Li C, Prince E, Tan B, Qing Y, Qin X, Shen C, Xue M, Zhou K, Chen Z, Xue J, Li W, Qin H, Wu X, Sun M, Nam Y, Chen CW, Huang W, Horne D, Rosen ST, He C, Chen J (2022) METTL16 exerts an m(6)A-independent function to facilitate translation and tumorigenesis. Nat Cell Biol 24:205–216. https://doi.org/10.1038/s41556-021-00835-2
Van Treeck B, Parker R (2018) Emerging roles for intermolecular RNA-RNA interactions in RNP assemblies. Cell 174:791–802. https://doi.org/10.1016/j.cell.2018.07.023
Varner EL, Trefely S, Bartee D, von Krusenstiern E, Izzo L, Bekeova C, O’Connor RS, Seifert EL, Wellen KE, Meier JL, Snyder NW (2020) Quantification of lactoyl-CoA (lactyl-CoA) by liquid chromatography mass spectrometry in mammalian cells and tissues. Open Biol 10:200187. https://doi.org/10.1098/rsob.200187
Vujic A, Lerchenmuller C, Wu TD, Guillermier C, Rabolli CP, Gonzalez E, Senyo SE, Liu X, Guerquin-Kern JL, Steinhauser ML, Lee RT, Rosenzweig A (2018) Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun 9:1659. https://doi.org/10.1038/s41467-018-04083-1
Wang C, Hou X, Guan Q, Zhou H, Zhou L, Liu L, Liu J, Li F, Li W, Liu H (2023) RNA modification in cardiovascular disease: implications for therapeutic interventions. Signal Transduct Target Ther 8:412. https://doi.org/10.1038/s41392-023-01638-7
Wang H, Maimaitiaili R, Yao J, Xie Y, Qiang S, Hu F, Li X, Shi C, Jia P, Yang H, Wei M, Zhao J, Zhou Z, Xie J, Jiang J, Cai H, Sluijter JPG, Xu Y, Zhang Y, Xiao J (2021) Percutaneous intracoronary delivery of plasma extracellular vesicles protects the myocardium against ischemia-reperfusion injury in canis. Hypertension 78:1541–1554. https://doi.org/10.1161/HYPERTENSIONAHA.121.17574
Wang JY, Lu AQ (2021) The biological function of m6A reader YTHDF2 and its role in human disease. Cancer Cell Int 21:109. https://doi.org/10.1186/s12935-021-01807-0
Wang K, Li Y, Qiang T, Chen J, Wang X (2021) Role of epigenetic regulation in myocardial ischemia/reperfusion injury. Pharmacol Res 170:105743. https://doi.org/10.1016/j.phrs.2021.105743
Wang L, Feng J, Feng X, Meng D, Zhao X, Wang J, Yu P, Xu GE, Hu M, Wang T, Lehmann HI, Li G, Sluijter JPG, Xiao J (2023) Exercise-induced circular RNA circUtrn is required for cardiac physiological hypertrophy and prevents myocardial ischaemia-reperfusion injury. Cardiovasc Res 119:2638–2652. https://doi.org/10.1093/cvr/cvad161
Wang L, Wang J, Yu P, Feng J, Xu GE, Zhao X, Wang T, Lehmann HI, Li G, Sluijter JPG, Xiao J (2022) METTL14 is required for exercise-induced cardiac hypertrophy and protects against myocardial ischemia-reperfusion injury. Nat Commun 13:6762. https://doi.org/10.1038/s41467-022-34434-y
Wang N, Wang W, Wang X, Mang G, Chen J, Yan X, Tong Z, Yang Q, Wang M, Chen L, Sun P, Yang Y, Cui J, Yang M, Zhang Y, Wang D, Wu J, Zhang M, Yu B (2022) Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ Res 131:893–908. https://doi.org/10.1161/CIRCRESAHA.122.320488
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C (2014) N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505:117–120. https://doi.org/10.1038/nature12730
Wei X, Huo Y, Pi J, Gao Y, Rao S, He M, Wei Q, Song P, Chen Y, Lu D, Song W, Liang J, Xu L, Wang H, Hong G, Guo Y, Si Y, Xu J, Wang X, Ma Y, Yu S, Zou D, Jin J, Wang F, Yu J (2022) METTL3 preferentially enhances non-m(6)A translation of epigenetic factors and promotes tumourigenesis. Nat Cell Biol 24:1278–1290. https://doi.org/10.1038/s41556-022-00968-y
Wu G, Zhang X, Gao F (2020) The epigenetic landscape of exercise in cardiac health and disease. J Sport Health Sci 10:648–659. https://doi.org/10.1016/j.jshs.2020.12.003
Wu TP, Wang T, Seetin MG, Lai Y, Zhu S, Lin K, Liu Y, Byrum SD, Mackintosh SG, Zhong M, Tackett A, Wang G, Hon LS, Fang G, Swenberg JA, Xiao AZ (2016) DNA methylation on N(6)-adenine in mammalian embryonic stem cells. Nature 532:329–333. https://doi.org/10.1038/nature17640
Xavier VJ, Martinou JC (2021) RNA granules in the mitochondria and their organization under mitochondrial stresses. Int J Mol Sci. https://doi.org/10.3390/ijms22179502
Xiao J, Rosenzweig A (2021) Exercise and cardiovascular protection: Update and future. J Sport Health Sci 10:607–608. https://doi.org/10.1016/j.jshs.2021.11.001
Xu H, Wang Z, Chen M, Zhao W, Tao T, Ma L, Ni Y, Li W (2021) YTHDF2 alleviates cardiac hypertrophy via regulating Myh7 mRNA decoy. Cell Biosci 11:132. https://doi.org/10.1186/s13578-021-00649-7
Xu P, Hu K, Zhang P, Sun ZG, Zhang N (2022) Hypoxia-mediated YTHDF2 overexpression promotes lung squamous cell carcinoma progression by activation of the mTOR/AKT axis. Cancer Cell Int 22:13. https://doi.org/10.1186/s12935-021-02368-y
Yang P, Mathieu C, Kolaitis RM, Zhang P, Messing J, Yurtsever U, Yang Z, Wu J, Li Y, Pan Q, Yu J, Martin EW, Mittag T, Kim HJ, Taylor JP (2020) G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181(325–345):e328. https://doi.org/10.1016/j.cell.2020.03.046
Yang S, Wei J, Cui YH, Park G, Shah P, Deng Y, Aplin AE, Lu Z, Hwang S, He C, He YY (2019) m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat Commun 10:2782. https://doi.org/10.1038/s41467-019-10669-0
Yang Y, Mbikyo MB, Zhang J, Zhang Y, Zhang N, Li Z (2022) The lncRNA MIAT regulates CPT-1a mediated cardiac hypertrophy through m(6)A RNA methylation reading protein Ythdf2. Cell Death Discov 8:167. https://doi.org/10.1038/s41420-022-00977-8
Yang Y, Yan Y, Yin J, Tang N, Wang K, Huang L, Hu J, Feng Z, Gao Q, Huang A (2023) O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N(6)-methyladenosine-dependent manner. Signal Transduct Target Ther 8:63. https://doi.org/10.1038/s41392-023-01316-8
Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S, Shen X, Wu Y, Zhang S, Wang X, Qiu S, Zhou J, Fan J, Huang H, Gao Q (2023) Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat Metab 5:61–79. https://doi.org/10.1038/s42255-022-00710-w
Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X, Jia R (2021) Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol 22:85. https://doi.org/10.1186/s13059-021-02308-z
Yu P, Wang J, Xu GE, Zhao X, Cui X, Feng J, Sun J, Wang T, Spanos M, Lehmann HI, Li G, Xu J, Wang L, Xiao J (2023) RNA m(6)A-regulated circ-ZNF609 suppression ameliorates doxorubicin-induced cardiotoxicity by upregulating FTO. JACC Basic Transl Sci 8:677–698. https://doi.org/10.1016/j.jacbts.2022.12.005
Zhang B, Jiang H, Dong Z, Sun A, Ge J (2021) The critical roles of m6A modification in metabolic abnormality and cardiovascular diseases. Genes Dis 8:746–758. https://doi.org/10.1016/j.gendis.2020.07.011
Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, Ding J, Czyz D, Hu R, Ye Z, He M, Zheng YG, Shuman HA, Dai L, Ren B, Roeder RG, Becker L, Zhao Y (2019) Metabolic regulation of gene expression by histone lactylation. Nature 574:575–580. https://doi.org/10.1038/s41586-019-1678-1
Zhong L, Liao D, Zhang M, Zeng C, Li X, Zhang R, Ma H, Kang T (2019) YTHDF2 suppresses cell proliferation and growth via destabilizing the EGFR mRNA in hepatocellular carcinoma. Cancer Lett 442:252–261. https://doi.org/10.1016/j.canlet.2018.11.006
Zou Z, Sepich-Poore C, Zhou X, Wei J, He C (2023) The mechanism underlying redundant functions of the YTHDF proteins. Genome Biol 24:17. https://doi.org/10.1186/s13059-023-02862-8
Acknowledgements
This work was supported by the grants from National Natural Science Foundation of China (82020108002 and 82225005 to JJ Xiao, 82270291 to LJ Wang), Science and Technology Commission of Shanghai Municipality (23410750100, 20DZ2255400 and 21XD1421300 to JJ Xiao), Natural Science Foundation of Shanghai (23ZR1423000 to LJ Wang).
Author information
Authors and Affiliations
Contributions
J.J.X., L.J.W., J.H.X. conceived the idea, designed the study, and instructed all experiments. L.J.W. drafted the manuscript. G.E.X., P.J.Y., Y.X.H., W.S.W., K.T.S., X.X.C., J.Q.W., T.H.W., and C.Y.C., performed the experiments and analyzed the data. E. C., G.P. L., D. C., and J. PG S. provided technical assistance, revised the manuscript, and polished the language. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Ge., Yu, P., Hu, Y. et al. Exercise training decreases lactylation and prevents myocardial ischemia–reperfusion injury by inhibiting YTHDF2. Basic Res Cardiol (2024). https://doi.org/10.1007/s00395-024-01044-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-024-01044-2