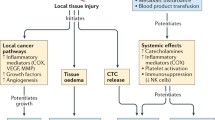
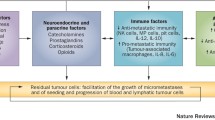
Abstract
Malignant tumors are characterized by their ability to metastasize, which is the main cause of cancer-related mortality. Besides intrinsic alternations in cancer cells, the tumor microenvironment plays a pivotal role in tumor growth and metastasis. Ample evidence suggests that the perioperative period and the excision of the primary tumor can promote the development of metastases and can influence long-term cancer patient outcomes. The role of cancer biology and its impact on the perioperative period are of increasing interest. This review will present evidence regarding fundamental principles of cancer biology, especially tumor microenvironment, and discuss new therapeutic opportunities in the perioperative timeframe. We will also discuss the regulatory signaling that could be relevant to various aspects of surgery and surgical responses, which could facilitate the metastatic process by directly or indirectly affecting malignant tissues and the tumor microenvironment. We address the influences of surgery-related stress, anesthetic and analgesic agents, blood transfusion, hypothermia, and β-adrenergic blockade administration on tumor growth and metastasis. Through an improved understanding of these processes, we will provide suggestions for potential new perioperative approaches aimed at improving treatment outcomes of cancer patients.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
•• Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. This paper provides a very important summary of the key factors that play a role in promoting cancer growth and progression.
Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–91.
•• Horowitz M, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12(4):213–26. This paper summarizes various opportunities in the perioperative time period.
Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011;89(4):557–63.
Dalton HJ, et al. Monocyte subpopulations in angiogenesis. Cancer Res. 2014;74(5):1287–93.
Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–12.
Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014;74(10):2663–8.
DeNardo DG, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67.
Ong SM, et al. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur J Immunol. 2012;42(1):89–100.
Chanmee T, et al. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6(3):1670–90.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–52.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51.
Su S, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–20.
Tjiu JW, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129(4):1016–25.
Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4.
Gooden MJ, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103.
Loi S, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–50.
Swartz MA, et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72(10):2473–80.
Reissfelder C, et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125(2):739–51.
Gottschalk A, et al. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110(6):1636–43.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61.
Liu S, et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. 2014;16(5):432.
Yuan XL, et al. Gastric cancer cells induce human CD4+Foxp3+ regulatory T cells through the production of TGF-beta1. World J Gastroenterol. 2011;17(15):2019–27.
Di Mitri D, et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515(7525):134–7.
Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol. 2014;41(2):174–84.
Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–506.
Chouaib S, et al. Endothelial cells as key determinants of the tumor microenvironment: interaction with tumor cells, extracellular matrix and immune killer cells. Crit Rev Immunol. 2010;30(6):529–45.
Franses JW, et al. Stromal endothelial cells directly influence cancer progression. Sci Transl Med. 2011;3(66):66ra5.
Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31(1–2):195–208.
Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48.
Heaney A, Buggy DJ. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth. 2012;109(Suppl 1):i17–28.
Kavanagh T, Buggy DJ. Can anaesthetic technique effect postoperative outcome? Curr Opin Anaesthesiol. 2012;25(2):185–98.
Cassinello F, et al. Cancer surgery: how may anesthesia influence outcome? J Clin Anesth. 2015;27(3):262–72.
Hiller J, Brodner G, Gottschalk A. Understanding clinical strategies that may impact tumour growth and metastatic spread at the time of cancer surgery. Best Pract Res Clin Anaesthesiol. 2013;27(4):427–39.
Lee JW, et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15(8):2695–702.
Vogelaar FJ, et al. Impact of anaesthetic technique on survival in colon cancer: a review of the literature. Gastroenterol Rep (Oxf). 2015. doi:https://doi.org/10.1093/gastro/gov001.
Melamed R, et al. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97(5):1331–9.
Shapiro J, et al. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J Clin Invest. 1981;68(3):678–85.
Tavare AN, et al. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130(6):1237–50.
Sessler DI, et al. Can regional analgesia reduce the risk of recurrence after breast cancer? Methodology of a multicenter randomized trial. Contemp Clin Trials. 2008;29(4):517–26.
Sylla P, Kirman I, Whelan RL. Immunological advantages of advanced laparoscopy. Surg Clin North Am. 2005;85(1):1–18, vii.
Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10(8):972–92.
Lennard TW, et al. The influence of surgical operations on components of the human immune system. Br J Surg. 1985;72(10):771–6.
Takabayashi A, et al. Change in mitochondrial membrane potential in peripheral blood lymphocytes, especially in natural killer cells, is a possible marker for surgical stress on the immune system. World J Surg. 2003;27(6):659–65.
Evans C, et al. Impact of surgery on immunologic function: comparison between minimally invasive techniques and conventional laparotomy for surgical resection of colorectal tumors. Am J Surg. 2009;197(2):238–45.
Tai LH, et al. A mouse tumor model of surgical stress to explore the mechanisms of postoperative immunosuppression and evaluate novel perioperative immunotherapies. J Vis Exp. 2014;85:e51253. doi:https://doi.org/10.3791/51253
Seth R, et al. Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Ann Surg. 2013;258(1):158–68.
Sammour T, et al. The humoral response after laparoscopic versus open colorectal surgery: a meta-analysis. J Surg Res. 2010;164(1):28–37.
Torres A, et al. Cytokine response in the postoperative period after surgical treatment of benign adnexal masses: comparison between laparoscopy and laparotomy. Surg Endosc. 2007;21(10):1841–8.
Wichmann MW, et al. Immunological effects of laparoscopic vs open colorectal surgery: a prospective clinical study. Arch Surg. 2005;140(7):692–7.
Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85(1):109–17.
Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–25.
Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22(3):263–77.
Melamed R, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–26.
Goldfarb Y, Ben-Eliyahu S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis. 2006;26:99–114.
Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6(12):1863–81.
Page GG. Surgery-induced immunosuppression and postoperative pain management. AACN Clin Issues. 2005;16(3):302–9 (quiz 416–8).
Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;(1):CD005033.
Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106–15.
Perez-Sayans M, et al. Beta-adrenergic receptors in cancer: therapeutic implications. Oncol Res. 2010;19(1):45–54.
Wu WK, et al. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295(1):7–16.
Mathew B, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112(3):558–67.
van der Bij GJ, et al. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg. 2009;249(5):727–34.
Masur K, et al. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61(7):2866–9.
Kerros C, et al. Reduction of cell proliferation and potentiation of Fas-induced apoptosis by the selective kappa-opioid receptor agonist U50 488 in the multiple myeloma LP-1 cells. J Neuroimmunol. 2010;220(1–2):69–78.
Sood AK, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010;120(5):1515–23.
Thaker PH, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–44.
Traynor C, Hall GM. Endocrine and metabolic changes during surgery: anaesthetic implications. Br J Anaesth. 1981;53(2):153–60.
Amato AC, Pescatori M. Effect of perioperative blood transfusions on recurrence of colorectal cancer: meta-analysis stratified on risk factors. Dis Colon Rectum. 1998;41(5):570–85.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209–15.
Rajagopalan S, et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108(1):71–7.
Ben-Eliyahu S, et al. Hypothermia in barbiturate-anesthetized rats suppresses natural killer cell activity and compromises resistance to tumor metastasis: a role for adrenergic mechanisms. Anesthesiology. 1999;91(3):732–40.
Vallianou NG, et al. Statins and cancer. Anticancer Agents Med Chem. 2014;14(5):706–12.
Bauchat JR, Habib AS. Evidence-based anesthesia for major gynecologic surgery. Anesthesiol Clin. 2015;33(1):173–207.
Acknowledgments
We thank the following for funding support: United States National Institutes of Health (CA109298, P50CA083639, P50CA098258, UH2 TR000943, CA177909), CPRIT RP140106, RP110595, the Department of Defense (OC073399, OC120547); the Blanton-Davis Ovarian Cancer Research Program; a Program Project Development Grant from the Ovarian Cancer Research Fund, Inc. and the Betty Anne Asche Murray Distinguished Professorship. Dr. Bernhard Riedel wishes to thank Drs. Donal Buggy, Vijaya Gottumukkala, and Erica Sloan for their kind assistance in the development of this issue and the reviewing of the articles.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Cancer Anesthesia.
Rights and permissions
About this article
Cite this article
Jiang, L., Nick, A.M. & Sood, A.K. Fundamental Principles of Cancer Biology: Does It Have Relevance to the Perioperative Period?. Curr Anesthesiol Rep 5, 250–256 (2015). https://doi.org/10.1007/s40140-015-0122-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-015-0122-9