Abstract
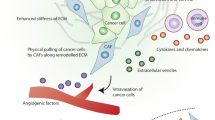
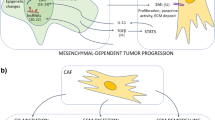
Several recent papers have now provided compelling experimental evidence that the progression of tumours towards a malignant phenotype does not depend exclusively on the cell-autonomous properties of cancer cells themselves but is also deeply influenced by tumour stroma reactivity, thereby undergoing a strict environmental control. Tumour microenvironmental elements include structural components such as the extracellular matrix or hypoxia as well as stromal cells, either resident cells or recruited from circulating precursors, as macrophages and other inflammatory cells, endothelial cells and cancer-associated fibroblasts (CAFs). All these elements synergistically play a specific role in cancer progression. This review summarizes our current knowledge on the role of CAFs in tumour progression, with a particular focus on the biunivocal interplay between CAFs and cancer cells leading to the activation of the epithelial–mesenchymal transition programme and the achievement of stem cell traits, as well as to the metabolic reprogramming of both stromal and cancer cells. Recent advances on the role of CAFs in the preparation of metastatic niche, as well as the controversial origin of CAFs, are discussed in light of the new emerging therapeutic implications of targeting CAFs.





Similar content being viewed by others
References
Gabbiani, G., Ryan, G. B., & Majne, G. (1971). Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia, 27(5), 549–550.
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C., & Brown, R. A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Reviews. Molecular Cell Biology, 3(5), 349–363.
Desmouliere, A., Redard, M., Darby, I., & Gabbiani, G. (1995). Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. American Journal of Pathology, 146(1), 56–66.
Rasanen, K., & Vaheri, A. (2010). Activation of fibroblasts in cancer stroma. Experimental Cell Research, 316(17), 2713–2722.
Dvorak, H. F. (1986). Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England Journal of Medicine, 315(26), 1650–1659.
Kalluri, R., & Zeisberg, M. (2006). Fibroblasts in cancer. Nature Reviews. Cancer, 6(5), 392–401.
Pietras, K., & Ostman, A. (2010). Hallmarks of cancer: Interactions with the tumor stroma. Experimental Cell Research, 316(8), 1324–1331.
Micke, P., & Ostman, A. (2004). Tumour–stroma interaction: Cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer, 45(Suppl 2), S163–S175.
O’Brien, P., & O’Connor, B. F. (2008). Seprase: An overview of an important matrix serine protease. Biochimica et Biophysica Acta, 1784(9), 1130–1145.
Hasebe, T., Tamura, N., Okada, N., Hojo, T., Akashi-Tanaka, S., Shimizu, C., et al. (2010). p53 expression in tumor-stromal fibroblasts is closely associated with the nodal metastasis and outcome of patients with invasive ductal carcinoma who received neoadjuvant therapy. Human Pathology, 41(2), 262–270.
Nakao, M., Ishii, G., Nagai, K., Kawase, A., Kenmotsu, H., Kon-No, H., et al. (2009). Prognostic significance of carbonic anhydrase IX expression by cancer-associated fibroblasts in lung adenocarcinoma. Cancer, 115(12), 2732–2743.
Utispan, K., Thuwajit, P., Abiko, Y., Charngkaew, K., Paupairoj, A., Chau-in, S., et al. (2010). Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Molecular Cancer, 9, 13.
Witkiewicz, A. K., Dasgupta, A., Sotgia, F., Mercier, I., Pestell, R. G., Sabel, M., et al. (2009). An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. American Journal of Pathology, 174(6), 2023–2034.
Yamanashi, T., Nakanishi, Y., Fujii, G., Akishima-Fukasawa, Y., Moriya, Y., Kanai, Y., et al. (2009). Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology, 77(1), 53–62.
Trimboli, A. J., Cantemir-Stone, C. Z., Li, F., Wallace, J. A., Merchant, A., Creasap, N., et al. (2009). Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature, 461(7267), 1084–1091.
Hill, R., Song, Y., Cardiff, R. D., & van, D. T. (2005). Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell, 123(6), 1001–1011.
Kiaris, H., Chatzistamou, I., Trimis, G., Frangou-Plemmenou, M., Pafiti-Kondi, A., & Kalofoutis, A. (2005). Evidence for nonautonomous effect of p53 tumor suppressor in carcinogenesis. Cancer Research, 65(5), 1627–1630.
Anderberg, C., & Pietras, K. (2009). On the origin of cancer-associated fibroblasts. Cell Cycle, 8(10), 1461–1462.
Hinz, B., Phan, S. H., Thannickal, V. J., Galli, A., Bochaton-Piallat, M. L., & Gabbiani, G. (2007). The myofibroblast: One function, multiple origins. American Journal of Pathology, 170(6), 1807–1816.
McAnulty, R. J. (2007). Fibroblasts and myofibroblasts: Their source, function and role in disease. The International Journal of Biochemistry & Cell Biology, 39(4), 666–671.
Ostman, A., & Augsten, M. (2009). Cancer-associated fibroblasts and tumor growth—Bystanders turning into key players. Current Opinion in Genetics and Development, 19(1), 67–73.
De, W. O., & Mareel, M. (2003). Role of tissue stroma in cancer cell invasion. The Journal of Pathology, 200(4), 429–447.
Giannoni, E., Bianchini, F., Masieri, L., Serni, S., Torre, E., Calorini, L., et al. (2010). Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial–mesenchymal transition and cancer stemness. Cancer Research, 70(17), 6945–6956.
Lohr, M., Schmidt, C., Ringel, J., Kluth, M., Muller, P., Nizze, H., et al. (2001). Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Research, 61(2), 550–555.
Bronzert, D. A., Pantazis, P., Antoniades, H. N., Kasid, A., Davidson, N., Dickson, R. B., et al. (1987). Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proceedings of the National Academy of Sciences of the United States of America, 84(16), 5763–5767.
Shao, Z. M., Nguyen, M., & Barsky, S. H. (2000). Human breast carcinoma desmoplasia is PDGF initiated. Oncogene, 19(38), 4337–4345.
Strutz, F., Zeisberg, M., Hemmerlein, B., Sattler, B., Hummel, K., Becker, V., et al. (2000). Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney International, 57(4), 1521–1538.
Cat, B., Stuhlmann, D., Steinbrenner, H., Alili, L., Holtkotter, O., Sies, H., et al. (2006). Enhancement of tumor invasion depends on transdifferentiation of skin fibroblasts mediated by reactive oxygen species. Journal of Cell Science, 119(Pt 13), 2727–2738.
Stuhlmann, D., Steinbrenner, H., Wendlandt, B., Mitic, D., Sies, H., & Brenneisen, P. (2004). Paracrine effect of TGF-beta1 on downregulation of gap junctional intercellular communication between human dermal fibroblasts. Biochemical and Biophysical Research Communications, 319(2), 321–326.
Giannoni, E., Bianchini, F., Calorini, L., & Chiarugi, P. (2011). Cancer associated fibroblasts exploit reactive oxygen species through a pro-inflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxidand & Redox Signaling, 14, 2361–2371.
Toullec, A., Gerald, D., Despouy, G., Bourachot, B., Cardon, M., Lefort, S., et al. (2010). Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Molecular Medicine, 2(6), 211–230.
Georges, P. C., & Janmey, P. A. (2005). Cell type-specific response to growth on soft materials. Journal of Applied Physiology, 98(4), 1547–1553.
Discher, D. E., Janmey, P., & Wang, Y. L. (2005). Tissue cells feel and respond to the stiffness of their substrate. Science, 310(5751), 1139–1143.
Assoian, R. K., & Klein, E. A. (2008). Growth control by intracellular tension and extracellular stiffness. Trends in Cell Biology, 18(7), 347–352.
Paszek, M. J., Zahir, N., Johnson, K. R., Lakins, J. N., Rozenberg, G. I., Gefen, A., et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell, 8(3), 241–254.
Chun, T. H., Hotary, K. B., Sabeh, F., Saltiel, A. R., Allen, E. D., & Weiss, S. J. (2006). A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell, 125(3), 577–591.
Huijbers, I. J., Iravani, M., Popov, S., Robertson, D., Al-Sarraj, S., Jones, C., et al. (2010). A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One, 5(3), e9808.
Kauppila, S., Stenback, F., Risteli, J., Jukkola, A., & Risteli, L. (1998). Aberrant type I and type III collagen gene expression in human breast cancer in vivo. The Journal of Pathology, 186(3), 262–268.
Hasebe, T., Sasaki, S., Imoto, S., Mukai, K., Yokose, T., & Ochiai, A. (2002). Prognostic significance of fibrotic focus in invasive ductal carcinoma of the breast: A prospective observational study. Modern Pathology, 15(5), 502–516.
Kaplan, R. N., Riba, R. D., Zacharoulis, S., Bramley, A. H., Vincent, L., Costa, C., et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature, 438(7069), 820–827.
Levental, K. R., Yu, H., Kass, L., Lakins, J. N., Egeblad, M., Erler, J. T., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell, 139(5), 891–906.
Santhanam, A. N., Baker, A. R., Hegamyer, G., Kirschmann, D. A., & Colburn, N. H. (2010). Pdcd4 repression of lysyl oxidase inhibits hypoxia-induced breast cancer cell invasion. Oncogene, 29(27), 3921–3932.
Olive, K. P., Jacobetz, M. A., Davidson, C. J., Gopinathan, A., McIntyre, D., Honess, D., et al. (2009). Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science, 324(5933), 1457–1461.
Shieh, A. C., Rozansky, H. A., Hinz, B., & Swartz, M. A. (2011). Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Research, 71(3), 790–800.
Gaggioli, C., Hooper, S., Hidalgo-Carcedo, C., Grosse, R., Marshall, J. F., Harrington, K., et al. (2007). Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature Cell Biology, 9(12), 1392–1400.
Plow, E. F., Haas, T. A., Zhang, L., Loftus, J., & Smith, J. W. (2000). Ligand binding to integrins. Journal of Biological Chemistry, 275(29), 21785–21788.
Velling, T., Risteli, J., Wennerberg, K., Mosher, D. F., & Johansson, S. (2002). Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. Journal of Biological Chemistry, 277(40), 37377–37381.
Pankov, R., & Yamada, K. M. (2002). Fibronectin at a glance. Journal of Cell Science, 115(Pt 20), 3861–3863.
Chen, S. H., Lin, C. Y., Lee, L. T., Chang, G. D., Lee, P. P., Hung, C. C., et al. (2010). Up-regulation of fibronectin and tissue transglutaminase promotes cell invasion involving increased association with integrin and MMP expression in A431 cells. Anticancer Research, 30(10), 4177–4186.
Mitra, A. K., Sawada, K., Tiwari, P., Mui, K., Gwin, K., & Lengyel, E. (2011). Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene, 30(13), 1566–1576.
Kobayashi, N., Miyoshi, S., Mikami, T., Koyama, H., Kitazawa, M., Takeoka, M., et al. (2010). Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Research, 70(18), 7073–7083.
Wang, W., Li, Q., Yamada, T., Matsumoto, K., Matsumoto, I., Oda, M., et al. (2009). Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clinical Cancer Research, 15(21), 6630–6638.
Jedeszko, C., Victor, B. C., Podgorski, I., & Sloane, B. F. (2009). Fibroblast hepatocyte growth factor promotes invasion of human mammary ductal carcinoma in situ. Cancer Research, 69(23), 9148–9155.
Matsumoto, K., & Nakamura, T. (2006). Hepatocyte growth factor and the Met system as a mediator of tumor–stromal interactions. International Journal of Cancer, 119(3), 477–483.
Matsumoto, K., Okazaki, H., & Nakamura, T. (1995). Novel function of prostaglandins as inducers of gene expression of HGF and putative mediators of tissue regeneration. Journal of Biochemistry, 117(2), 458–464.
Bhowmick, N. A., Chytil, A., Plieth, D., Gorska, A. E., Dumont, N., Shappell, S., et al. (2004). TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science, 303(5659), 848–851.
Gerber, P. A., Hippe, A., Buhren, B. A., Muller, A., & Homey, B. (2009). Chemokines in tumor-associated angiogenesis. Biological Chemistry, 390(12), 1213–1223.
Matsuo, Y., Ochi, N., Sawai, H., Yasuda, A., Takahashi, H., Funahashi, H., et al. (2009). CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. International Journal of Cancer, 124(4), 853–861.
Orimo, A., Gupta, P. B., Sgroi, D. C., Arenzana-Seisdedos, F., Delaunay, T., Naeem, R., et al. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell, 121(3), 335–348.
Augsten, M., Hagglof, C., Olsson, E., Stolz, C., Tsagozis, P., Levchenko, T., et al. (2009). CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proceedings of the National Academy of Sciences of the United States of America, 106(9), 3414–3419.
Erez, N., Truitt, M., Olson, P., Arron, S. T., & Hanahan, D. (2010). Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell, 17(2), 135–147.
Hynes, R. O. (2009). The extracellular matrix: Not just pretty fibrils. Science, 326(5957), 1216–1219.
Roy, R., Yang, J., & Moses, M. A. (2009). Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. Journal of Clinical Oncology, 27(31), 5287–5297.
Vosseler, S., Lederle, W., Airola, K., Obermueller, E., Fusenig, N. E., & Mueller, M. M. (2009). Distinct progression-associated expression of tumor and stromal MMPs in HaCaT skin SCCs correlates with onset of invasion. International Journal of Cancer, 125(10), 2296–2306.
Lederle, W., Hartenstein, B., Meides, A., Kunzelmann, H., Werb, Z., Angel, P., et al. (2010). MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis, 31(7), 1175–1184.
Dean, J. P., & Nelson, P. S. (2008). Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. British Journal of Cancer, 98(2), 245–249.
Blasi, F., & Sidenius, N. (2010). The urokinase receptor: Focused cell surface proteolysis, cell adhesion and signaling. FEBS Letters, 584(9), 1923–1930.
Noskova, V., Ahmadi, S., Asander, E., & Casslen, B. (2009). Ovarian cancer cells stimulate uPA gene expression in fibroblastic stromal cells via multiple paracrine and autocrine mechanisms. Gynecologic Oncology, 115(1), 121–126.
Coppe, J. P., Desprez, P. Y., Krtolica, A., & Campisi, J. (2010). The senescence-associated secretory phenotype: The dark side of tumor suppression. Annual Review of Pathology, 5, 99–118.
Davalos, A. R., Coppe, J. P., Campisi, J., & Desprez, P. Y. (2010). Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Reviews, 29(2), 273–283.
Laberge, R. M., Awad, P., Campisi, J., & Desprez, P. Y. (2011). Epithelial–mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. doi:10.1007/s12307-011-0069-4.
Lee, C. (1996). Role of androgen in prostate growth and regression: Stromal–epithelial interaction. The Prostate. Supplement, 6, 52–56.
Chang, S. M., & Chung, L. W. (1989). Interaction between prostatic fibroblast and epithelial cells in culture: Role of androgen. Endocrinology, 125(5), 2719–2727.
Cano, P., Godoy, A., Escamilla, R., Dhir, R., & Onate, S. A. (2007). Stromal–epithelial cell interactions and androgen receptor–coregulator recruitment is altered in the tissue microenvironment of prostate cancer. Cancer Research, 67(2), 511–519.
Ricciardelli, C., Choong, C. S., Buchanan, G., Vivekanandan, S., Neufing, P., Stahl, J., et al. (2005). Androgen receptor levels in prostate cancer epithelial and peritumoral stromal cells identify non-organ confined disease. Prostate, 63(1), 19–28.
Henshall, S. M., Quinn, D. I., Lee, C. S., Head, D. R., Golovsky, D., Brenner, P. C., et al. (2001). Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Research, 61(2), 423–427.
Zhao, Y., Nichols, J. E., Valdez, R., Mendelson, C. R., & Simpson, E. R. (1996). Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Molecular Endocrinology, 10(11), 1350–1357.
Simpson, E. R., & Davis, S. R. (2001). Minireview: Aromatase and the regulation of estrogen biosynthesis—Some new perspectives. Endocrinology, 142(11), 4589–4594.
Santen, R. J., Santner, S. J., Pauley, R. J., Tait, L., Kaseta, J., Demers, L. M., et al. (1997). Estrogen production via the aromatase enzyme in breast carcinoma: Which cell type is responsible? The Journal of Steroid Biochemistry and Molecular Biology, 61(3–6), 267–271.
Howell, A., Cuzick, J., Baum, M., Buzdar, A., Dowsett, M., Forbes, J. F., et al. (2005). Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet, 365(9453), 60–62.
Joyce, J. A., & Pollard, J. W. (2009). Microenvironmental regulation of metastasis. Nature Reviews. Cancer, 9(4), 239–252.
De, W. O., Demetter, P., Mareel, M., & Bracke, M. (2008). Stromal myofibroblasts are drivers of invasive cancer growth. International Journal of Cancer, 123(10), 2229–2238.
De Wever, O., Nguyen, Q. D., Van, H. L., Bracke, M., Bruyneel, E., Gespach, C., et al. (2004). Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. The FASEB Journal, 18(9), 1016–1018.
Kalluri, R. (2009). EMT: When epithelial cells decide to become mesenchymal-like cells. The Journal of Clinical Investigation, 119(6), 1417–1419.
Thiery, J. P., Acloque, H., Huang, R. Y., & Nieto, M. A. (2009). Epithelial–mesenchymal transitions in development and disease. Cell, 139(5), 871–890.
Blick, T., Hugo, H., Widodo, E., Waltham, M., Pinto, C., Mani, S. A., et al. (2010). Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. Journal of Mammary Gland Biology and Neoplasia, 15(2), 235–252.
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., et al. (2008). The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell, 133(4), 704–715.
Klarmann, G. J., Hurt, E. M., Mathews, L. A., Zhang, X., Duhagon, M. A., Mistree, T., et al. (2009). Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clinical & Experimental Metastasis, 26(5), 433–446.
Visvader, J. E., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nature Reviews. Cancer, 8(10), 755–768.
Liao, C. P., Adisetiyo, H., Liang, M., & Roy-Burman, P. (2010). Cancer-associated fibroblasts enhance the gland-forming capability of prostate cancer stem cells. Cancer Research, 70(18), 7294–7303.
Wu, Y., Deng, J., Rychahou, P. G., Qiu, S., Evers, B. M., & Zhou, B. P. (2009). Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell, 15(5), 416–428.
Radisky, D. C., Levy, D. D., Littlepage, L. E., Liu, H., Nelson, C. M., Fata, J. E., et al. (2005). Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature, 436(7047), 123–127.
De, W. O., Pauwels, P., De, C. B., Sabbah, M., Emami, S., Redeuilh, G., et al. (2008). Molecular and pathological signatures of epithelial–mesenchymal transitions at the cancer invasion front. Histochemistry and Cell Biology, 130(3), 481–494.
Patocs, A., Zhang, L., Xu, Y., Weber, F., Caldes, T., Mutter, G. L., et al. (2007). Breast-cancer stromal cells with TP53 mutations and nodal metastases. The New England Journal of Medicine, 357(25), 2543–2551.
Jones, R. G., & Thompson, C. B. (2009). Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes & Development, 23(5), 537–548.
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science, 324(5930), 1029–1033.
Christofk, H. R., Vander Heiden, M. G., Harris, M. H., Ramanathan, A., Gerszten, R. E., Wei, R., et al. (2008). The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature, 452(7184), 230–233.
Vander Heiden, M. G., Locasale, J. W., Swanson, K. D., Sharfi, H., Heffron, G. J., Amador-Noguez, D., et al. (2010). Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science, 329(5998), 1492–1499.
Luo, W., Hu, H., Chang, R., Zhong, J., Knabel, M., O’Meally, R., et al. (2011). Pyruvate kinase M2 Is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell, 145(5), 732–744.
Pavlides, S., Whitaker-Menezes, D., Castello-Cros, R., Flomenberg, N., Witkiewicz, A. K., Frank, P. G., et al. (2009). The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle, 8(23), 3984–4001.
Martinez-Outschoorn, U. E., Trimmer, C., Lin, Z., Whitaker-Menezes, D., Chiavarina, B., Zhou, J., et al. (2010). Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle, 9(17), 3515–3533.
Koukourakis, M. I., Giatromanolaki, A., Harris, A. L., & Sivridis, E. (2006). Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: A metabolic survival role for tumor-associated stroma. Cancer Research, 66(2), 632–637.
Garzon, R., Marcucci, G., & Croce, C. M. (2010). Targeting microRNAs in cancer: Rationale, strategies and challenges. Nature Reviews. Drug Discovery, 9(10), 775–789.
Tazawa, H., Kagawa, S., & Fujiwara, T. (2011). MicroRNAs as potential target gene in cancer gene therapy of gastrointestinal tumors. Expert Opinion on Biological Therapy, 11(2), 145–155.
Musumeci, M., Coppola, V., Addario, A., Patrizii, M., Maugeri-Sacca, M., Memeo, L., et al. (2011). Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene, 30, 4231–4242.
Nielsen, B. S., Jorgensen, S., Fog, J. U., Sokilde, R., Christensen, I. J., Hansen, U., et al. (2011). High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clinical & Experimental Metastasis, 28(1), 27–38.
Yao, Q., Cao, S., Li, C., Mengesha, A., Kong, B., & Wei, M. (2011). Micro-RNA-21 regulates TGF-beta-induced myofibroblast differentiation by targeting PDCD4 in tumor–stroma interaction. International Journal of Cancer, 128(8), 1783–1792.
Aprelikova, O., Yu, X., Palla, J., Wei, B. R., John, S., Yi, M., et al. (2010). The role of miR-31 and its target gene SATB2 in cancer-associated fibroblasts. Cell Cycle, 9(21), 4387–4398.
Lim, P. K., Bliss, S. A., Patel, S. A., Taborga, M., Dave, M. A., Gregory, L. A., et al. (2011). Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Research, 71(5), 1550–1560.
Grange, C., Tapparo, M., Collino, F., Vitillo, L., Damasco, C., Deregibus, M. C., et al. (2011). Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung pre-metastatic niche. Cancer Research, 71, 5346–5356.
Nguyen, D. X., Bos, P. D., & Massague, J. (2009). Metastasis: From dissemination to organ-specific colonization. Nature Reviews. Cancer, 9(4), 274–284.
Tu, S. M., Lin, S. H., & Logothetis, C. J. (2002). Stem-cell origin of metastasis and heterogeneity in solid tumours. The Lancet Oncology, 3(8), 508–513.
Psaila, B., & Lyden, D. (2009). The metastatic niche: Adapting the foreign soil. Nature Reviews. Cancer, 9(4), 285–293.
Duda, D. G., Duyverman, A. M., Kohno, M., Snuderl, M., Steller, E. J., Fukumura, D., et al. (2010). Malignant cells facilitate lung metastasis by bringing their own soil. Proceedings of the National Academy of Sciences of the United States of America, 107, 21677–21682.
Sung, S. Y., Hsieh, C. L., Law, A., Zhau, H. E., Pathak, S., Multani, A. S., et al. (2008). Coevolution of prostate cancer and bone stroma in three-dimensional coculture: Implications for cancer growth and metastasis. Cancer Research, 68(23), 9996–10003.
Pietras, K., Pahler, J., Bergers, G., & Hanahan, D. (2008). Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Medicine, 5(1), e19.
Wu, M. P., Young, M. J., Tzeng, C. C., Tzeng, C. R., Huang, K. F., Wu, L. W., et al. (2008). A novel role of thrombospondin-1 in cervical carcinogenesis: Inhibit stroma reaction by inhibiting activated fibroblasts from invading cancer. Carcinogenesis, 29(6), 1115–1123.
Wen, J., Matsumoto, K., Taniura, N., Tomioka, D., & Nakamura, T. (2004). Hepatic gene expression of NK4, an HGF-antagonist/angiogenesis inhibitor, suppresses liver metastasis and invasive growth of colon cancer in mice. Cancer Gene Therapy, 11(6), 419–430.
Kim, K. J., Wang, L., Su, Y. C., Gillespie, G. Y., Salhotra, A., Lal, B., et al. (2006). Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clinical Cancer Research, 12(4), 1292–1298.
Crawford, Y., Kasman, I., Yu, L., Zhong, C., Wu, X., Modrusan, Z., et al. (2009). PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell, 15(1), 21–34.
Sato, N., Maehara, N., & Goggins, M. (2004). Gene expression profiling of tumor–stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Research, 64(19), 6950–6956.
Hu, M., Peluffo, G., Chen, H., Gelman, R., Schnitt, S., & Polyak, K. (2009). Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proceedings of the National Academy of Sciences of the United States of America, 106(9), 3372–3377.
Mann, J., Oakley, F., Akiboye, F., Elsharkawy, A., Thorne, A. W., & Mann, D. A. (2007). Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: Implications for wound healing and fibrogenesis. Cell Death and Differentiation, 14(2), 275–285.
Scott, A. M., Wiseman, G., Welt, S., Adjei, A., Lee, F. T., Hopkins, W., et al. (2003). A phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clinical Cancer Research, 9(5), 1639–1647.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cirri, P., Chiarugi, P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev 31, 195–208 (2012). https://doi.org/10.1007/s10555-011-9340-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-011-9340-x