Abstract
Purpose of Review
Infants and young children with congenital heart disease and valvular lesions may require valve replacement when a durable repair is unlikely. The fundamental problem with currently available valve substitutes in all positions is the lack of somatic growth potential. Young patients are therefore committed to multiple reoperations for successively larger valve replacements by the time they reach adulthood.
Recent Findings
An emerging solution to this issue is allogeneic valve transplantation whereby the implanted valve is harvested from the heart of a deceased donor. The major advantage of this approach is the use of living tissue which grows adaptively with the child, thereby minimizing the number and additive risk of subsequent reoperations for valve exchange but incurring the risks of immunosuppression.
Summary
Here, we review the advantages and disadvantages of currently available valve replacement options for each of the four valves. We also discuss the potential role and future directions for allogeneic valve transplantation in pediatric valve surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital heart disease is the leading cause of newborn death due to congenital anomalies in the USA [1]. Of those surviving the newborn period, approximately 25% require surgery within the first year of life [2, 3], often for valvular malformations and outflow tract lesions. Valves and valved conduits from bioprosthetic and synthetic materials are traditionally used when repair is not feasible, though no ideal substitute exists. Besides degenerative, thromboembolic, and bleeding complications, the primary disadvantage is lack of growth potential, necessitating serial reoperations to exchange nonviable prostheses for larger sizes [4]. This causes considerable morbidity and mortality, with significant psychosocial impacts on children and families.
Allogeneic valve transplantation is an emerging therapy that involves the replacement of irreparable native valves with fresh, living allografts from a size-matched donor heart. Since valves are not cryopreserved or fixed, they can grow with the child. Despite the need for systemic immunosuppression to preserve allograft viability [5•], a handful of allogeneic valve transplants have been performed to date [6, 7•]. This review summarizes pediatric valve replacement options and comments on the history and potential of allogeneic valve transplantation to deliver a valve that will grow with the patient. Historical and contemporary valve replacement options are summarized in Table 1.
Aortic Valve Replacement (AVR)
Congenital aortic stenosis is present in approximately 5% of children with congenital heart disease [8] and often associated with a bicuspid valve and left ventricular outflow tract obstruction. While repairs are increasingly being performed with acceptable results [9,10,11], the optimal replacement option remains controversial. Contemporary AVR literature is summarized in Supplementary Table 1.
Ross Procedure
The Ross operation is an attractive option for children and young adults. The diseased valve is replaced with a pulmonary autograft and the right ventricular outflow tract (RVOT) is reconstructed using a cryopreserved or decellularized homograft. Advantages of the pediatric Ross operation include an excellent hemodynamic profile, avoidance of anticoagulation, and growth potential of the pulmonary autograft, though not the homograft [12, 13].
While technically complex, the Ross procedure is safe in children and adolescents, with operative mortality rates of approximately 1% [14•, 15•]. Several groups have also demonstrated excellent long-term survival and freedom from autograft reintervention in older children [16,17,18,19,20,21,22,23,24,25,26,27,28]. In a meta-analysis by Moroi and colleagues, late mortality was 0.04–1.83% per year, with annual reoperation rates for autograft failure of 0.37–2.81%. In neonates and infants, however, mortality and reintervention rates are much higher [15•]; a recent analysis of the Ross procedure using the Society of Thoracic Surgeons Congenital Heart Surgery database revealed an operative mortality of 1.5% and 0.8% in children and adolescents, respectively, compared to 24.1% and 11.2% in neonates and infants [14•]. Long-term outcomes also differ—Donald and colleagues demonstrated a 10-year survival of 78.9% in neonates and infants and 96.2% in children older than 1 year. Overall, 10-year freedom from autograft reoperation was 86%, with age younger than 1 year at operation being a risk factor [16].
The major shortcoming of the pediatric Ross operation is the need for reintervention on either outflow tract. Pathologic autograft dilation may occur at the level of the annulus, sinuses, or sinotubular junction, possibly from failure of the pulmonary autograft to adapt to higher systemic pressures [26, 28, 29]. Despite technical modifications to mitigate this risk [30, 31], these may inhibit somatic growth and are therefore not feasible in infants and young children. Reintervention on the right ventricle to pulmonary artery homograft is also common. Nelson and colleagues reported an overall 15-year freedom from homograft reintervention of 53%; younger age was significantly associated with homograft reintervention, with a 15-year freedom from reintervention of only 19% in neonates and infants [19]. As such, many argue that the Ross operation converts single valve disease into double valve surveillance. Nonetheless, the Ross operation is our preferred approach for AVRs in children and young adults given excellent survival, hemodynamic profile, and quality of life, with few valve-related adverse events [32•].
Mechanical Aortic Valve Replacement
Mechanical aortic valves are reserved for older children who are not Ross candidates due to connective tissue disorders or abnormal (or absent) pulmonary valves [33]. In the largest pediatric series, Myers and colleagues reported 5.5% operative mortality and 82% 10-year survival. Ten-year freedom from reoperation was 78%, with the most common reintervention reasons being pannus ingrowth and valve thrombosis and risk factors being younger age and implantation of the smallest prosthesis (16 mm) [34]. In a contemporary meta-analysis, the annual pooled mechanical AVR reoperation rate was 1.0% [35].
Though a reliable option for select patients [34, 36], a disadvantage of mechanical prostheses is the thromboembolism risk, necessitating lifelong anticoagulation. This can be challenging in children due to activity restraints, medication compliance, and use of anticoagulation in women of child-bearing age. In a recent meta-analysis, pooled annual rates of thromboembolism and major bleeding were 0.76% and 0.39%, respectively [35]. Furthermore, a root enlargement may be required to accommodate even the smallest mechanical prosthesis in neonates or infants. Finally, despite excellent durability, reoperation following mechanical AVR in children is still common—ranging from 55 to 90% at 15 years [12, 36,37,38]—with the main indications being patient-prosthesis mismatch, along with pannus formation causing subvalvar obstruction and endocarditis.
Bioprosthetic Aortic Valve Replacement
Tissue prostheses are commonly used in adults and allow for avoidance of chronic anticoagulation, though applications are limited in pediatrics. Prostheses are unavailable in sizes smaller than 19 mm and longevity is decreased in younger patients, in whom structural valve degeneration is typically accelerated [39,40,41,42,43]. Indeed, valve deterioration is 6–9 times more rapid in young adults compared to older patients, with an incidence of 30–50% at 10–20 years [44]. Saleeb and colleagues published a series of 73 pediatric patients (median age 18.8 years [range, 3.8–29.2]) who received a bioprosthetic AVR over a 15-year period. The authors noted significantly accelerated degeneration of the Mitroflow valve (Sorin Group Italia, Vercelli, Italy) compared to other tissue valves, with freedom from valve failure (explant or death) of only 20% versus 87.6% at 4 years. All explanted Mitroflows had heavy leaflet calcification and immobilization [45]. Finally, children receiving a bioprosthetic AVR will likely require multiple reoperations for patient-prosthesis mismatch due to fixed valve size.
Homograft Aortic Valve Replacement
Homografts are obtained from deceased donors and cryopreserved for prolonged storage. Though associated with excellent early hemodynamic profiles, homografts can exhibit rapid structural degeneration and failure. In pediatric patients undergoing homograft AVR, Fukushima and colleagues observed a 10-year freedom from structural valve degeneration of only 55%. Younger age was associated with structural failure [46], presumably due to higher cardiac output and a more active immune response in children [47,48,49]. Other groups reported similar rates of reintervention for homograft structural degeneration in young patients [18, 36, 48,49,50,51,52]. Wider use of aortic homografts is also restricted by the limited number of grafts in small sizes; homografts are thus generally utilized when the Ross is not feasible or when a mechanical AVR is not advisable [18].
Aortic Valve Neocuspidization (Ozaki Procedure)
The Ozaki procedure involves the replacement of diseased aortic valve cusps with tailored neocusps of autologous pericardium or synthetic patch material. Ozaki and colleagues first published on this technique in adults, with 10-year survival and freedom from reoperation of 86% and 95%, respectively [53]. The procedure has since been performed with acceptable short-term outcomes in children: Baird and colleagues reported a 2-year freedom from moderate or greater aortic regurgitation or stenosis of 88% and freedom from reoperation of 91% at 1.5 years in a cohort with a median age of 12.4 years [54].
Despite promising short- to mid-term outcomes in older children [54, 55], the long-term durability and eventual mode of failure remain unknown. Importantly, synthetic patch material clearly reduces durability [56], and observations of reduced leaflet motion have led to nearly half of Ozaki neocuspidization recipients requiring coumadin. Applications in younger children may thus be limited by this poor durability and lack of growth potential of synthetic material.
Pulmonary Valve Replacement (PVR)
The pulmonary valve is often replaced in congenital patients, with an increasing need for PVR as more survive to adulthood. Contemporary PVR literature is summarized in Supplementary Table 2.
Bioprosthetic Pulmonary Valve Replacement
Few have evaluated the optimal bioprosthetic PVR in pediatric patients [57,58,59,60,61,62], with modern understandings often extrapolated from the adult AVR experience. We analyzed the Inspiris Resilia valve (Edwards Lifsciences, Irvine, CA)—a prosthesis commonly used for AVR in adults—in the pulmonic position in children and young adults with congenital heart disease. Among propensity-matched patients, 2-year freedom from valve failure was lower in the Inspiris group compared to those who did not receive an Inspiris valve (53.5% vs. 78.5%, p = 0.03), with prosthetic regurgitation being the main mechanism of failure. Inspiris durability was also poorer when implanted in the native RVOT compared to as a conduit, with 18-month freedom from valve failure of 59.0% vs. 85.9% (p = 0.03) [57]. In another large, single-center study, Nomoto and colleagues showed good short-term outcomes among all studied bioprosthetic PVR options, though younger patients had almost fivefold greater risk of reintervention than adults (independent of valve type). This risk decreased by 10% for each increasing year of age at surgery [58]. Calderone and colleagues reported a 5-year freedom from valve replacement of 81%, with younger age associated with early prosthetic failure [59]. While the current bioprosthetic PVR strategy is oversizing to facilitate future valve-in-valve procedures, this is associated with structural valve deterioration and should be performed with caution [60].
Mechanical Pulmonary Valve Replacement
Mechanical valves are rarely used in the pulmonary position due to thrombosis concerns in the low pressure, right-sided system. Still, the risk profile is relatively favorable [61,62,63,64,65,66]. A multicenter retrospective analysis of 364 patients reported a freedom from valve thrombosis of 91% and 86% at 5 and 10 years, respectively. The annual incidence of valve thrombosis was 1.7%. Major bleeding complications were not reported. Durability was excellent, with 97% and 91% freedom from reintervention at 5 and 10 years, respectively [61]. As PVR patients have often had multiple prior sternotomies, a mechanical prosthesis may thus be reasonable to limit further interventions, though requires systemic anticoagulation.
Transcatheter Pulmonary Valve Replacement (TPVR)
Transcatheter pulmonary valve implantation is used to treat failing RVOT conduits or bioprosthetic pulmonary valves and is standard of care provided anatomy is favorable [67,68,69,70,71]. Available balloon-expandable valves include the Melody valve (Medtronic, Minneapolis, MN) made from stented bovine jugular vein and SAPIEN transcatheter bovine pericardial valve (Edwards Lifesciences, Irvine, CA). The Melody Investigational Device Exemption trial demonstrated an estimated 10-year survival of 90%, along with 79% freedom from RVOT reoperation and 60% freedom from any valve reintervention. Ten-year freedom from valve dysfunction was 53% and significantly lower in children than adults [68]. While this trial affirmed the role of TPVR technologies in the lifetime management of patients with repaired congenital heart disease and a dysfunctional RVOT conduit or pulmonary valve prosthesis, young patients inevitably outgrow the implant. Additionally, the risk of endocarditis appears to be higher with these devices.
Right Ventricle-to-Pulmonary Artery Conduits
Reconstruction of right ventricle-to-pulmonary artery (RV-PA) continuity is integral to congenital cardiac surgery repairs. Factors influencing the choice of conduit include original pathology, age, and availability. All conduits, however, do not grow, making serial reoperations unavoidable [72]. Contemporary RV-PA conduit literature is summarized in Supplementary Table 2.
Homografts
Pulmonary and aortic homografts have favorable handling properties, are available in sizes small enough for infants, and have low infection risk. Disadvantages, however, are lack of growth potential, risk of structural degeneration and calcification, high cost, short shelf-life (~ 2 years), and limited availability in sizes small enough for neonates or large enough for younger children. Furthermore, some patients may develop human leukocyte antibodies after homograft implantation and this sensitization may increase the risk for antibody-mediated rejection and graft dysfunction should the patient require a future heart transplant.
In regard to homograft durability, freedom from reintervention ranges widely—30 to over 80% at 10 years [73,74,75,76,77,78,79,80]—with smaller conduit size, younger age at operation, and a non-Ross operation being risk factors for conduit failure [75, 81, 82]. Several series have also demonstrated superior durability of pulmonary over aortic homografts [73, 81, 82]. Lewis and colleagues reported a series of 455 consecutive pediatric patients (mean age 6.4 ± 5.8 years) who underwent RV-PA conduit reconstruction with either a pulmonary homograft, aortic homograft, or bovine jugular vein graft and demonstrated a 10- and 28-year freedom from conduit replacement of 79.6% and 66.0%, respectively, for pulmonary homografts, compared to 49.8% and 23.0%, at 10 and 30 years, respectively, for aortic homografts [73]. Pseudoaneurysms and conduit dilation, however, are more common with pulmonary homografts [83].
Xenografts
Xenografts of bovine or porcine origin may be stented or non-stented. Advantages include abundant supply, availability in small sizes, favorable handling characteristics, and low cost compared to homografts [84]. One of the most used, the Contegra xenograft (Medtronic, Minneapolis, MN), is made from valved bovine jugular vein and small enough for infant RVOT reconstruction. Durability is at least comparable to pulmonary homografts [85,86,87,88,89], though younger age at implantation is a risk factor for reintervention and distal conduit stenosis [90]. Importantly, the Contegra homograft is associated with increased risk of endocarditis compared to other biological conduits [86].
Synthetic Valved Conduits
Composite valved conduits made of synthetic tube grafts with bioprosthetic or mechanical valves are available commercially or can be manually constructed [91]. Commercially available conduits include the Hancock (Medtronic, Minneapolis, MN), consisting of a porcine valve within a Dacron tube [92]. Advantages include prolonged shelf-life, widespread availability, and a rigid pericardial valve annulus resistant to sternal compression. Size limitations, however, limit use in neonates and small infants.
Mitral Valve Replacement (MVR)
Rheumatic heart disease, endocarditis, mitral stenosis, and failed atrioventricular septal defect repair are the most common indications for MVR in pediatric patients. Unfortunately, MVR carries the highest operative and long-term mortality risk of all pediatric valve replacements [93,94,95]. Contemporary MVR literature is summarized in Supplementary Table 3.
Mechanical and Bioprosthetic MVR
Surgical MVR operative mortality in infants and young children is 10 to 36% [94,95,96,97]. In a multi-institutional study of 139 patients under 5 years old, Calderone and colleagues reported 74% 10-year survival. Most deaths occurred shortly after initial MVR and 5-year freedom from reoperation was 81% among survivors. An increased ratio of valve size to patient weight was an age-adjusted predictor of death [94]. This is notable as valves are often oversized and implanted in a supra-annular position in infants and small children, leading to leaflet entrapment, left ventricular outflow tract obstruction, and atrioventricular block [94,95,96]. Other studies have also found both of these techniques to be associated with increased mortality [93, 98,99,100].
Despite oversizing, prosthetic valves must be replaced given lack of growth potential. Choi and colleagues reported freedom from redo MVR of 76% and 44% at 5 and 10 years, respectively; time to reoperation was associated with prosthesis type, with porcine and pericardial valves at greatest risk. Prosthesis type was also associated with time to death or transplant, with porcine valves at greater risk than mechanical valves [101•]. Still, mandatory anticoagulation and size limitations remain a disadvantage of mechanical valves.
Transcatheter MVR
Given poor outcomes and size limitations, stented bovine jugular vein valves (Melody valve, Medtronic, Minneapolis, MN), originally approved for TPVR, have been implanted in the mitral position as an off-label use [102]. Valves are initially surgically implanted into a small mitral annulus and serially balloon-dilated to accommodate somatic growth [103]—this technique has been employed as a bridge to a future fixed-diameter valve replacement with acceptable short- and mid-term outcomes [104, 105]. Choi and colleagues demonstrated that stented bovine jugular vein valves may exhibit greater durability than conventional xenograft prostheses in small children. All balloon dilations performed on Melody valves were successful in resolving or significantly decreasing the transmitral gradient, with only 11.8% of patients developing mitral regurgitation [101•]. While paravalvular leaks are common, this is likely related to surgical implantation technique and device design.
Tricuspid Valve Replacement (TVR)
TVR is rarely performed in young children, most often for irreparable Ebstein’s anomaly and tricuspid valve dysplasia. Reports on pediatric TVR consist of small series from single institutions, with early mortality of 9–36% [106,107,108,109]. Contemporary TVR literature is summarized in Supplementary Table 4.
Bioprosthetic and Mechanical Tricuspid Valve Replacement
Several adult studies have demonstrated acceptable tricuspid bioprosthesis durability [110,111,112], though few have been performed in children. Boyd and colleagues recently reported disappointing results with bioprosthetic TVR for Ebstein’s anomaly: compared to cone repair, the valve replacement group had a lower freedom from reoperation at 6 years (cone: 91% vs. TVR: 68%, p = 0.02) and worse right ventricular function at mean follow-up of 4 years [113]. In an older series, however, Kiziltan and colleagues reported favorable long-term outcomes in 158 patients with Ebstein’s anomaly or an Ebsteinoid valve undergoing TVR with a porcine bioprosthesis: 15-year survival and freedom from reoperation was 92.5% and 80.6%, respectively. Of note, bioprostheses demonstrated higher freedom from reintervention than mechanical prostheses [109]. Similarly, Bartlett and colleagues saw improved survival and lower rates of pacemaker requirements in children who received bioprostheses compared to mechanical TVR. Implantation of a large valve relative to the patient’s weight was a predictor of postoperative mortality, indicating that oversizing to delay replacement may impair ventricular dynamics and restrict leaflet mobility [107].
Overall, mechanical prostheses are rarely used in the tricuspid position due to valve thrombosis risk, with reported incidence of 3–18% [110, 114]. As such, anticoagulation with a higher International Normalized Ratio goal is required, significantly increasing the risk of bleeding complications.
Historical Use and Development of Viable Allograft/Homograft Valves
The initial orthotopic implantation of aortic valve allografts was reported in 1962 by Ross [115] and Barrett-Boyes [116]. Valves were collected at autopsy, minimally treated, and implanted within hours to days. Outcomes were favorable, with the New Zealand group reporting 79% survival and 13% incidence of valve failure at 6 years [117]. Over the next decades, multiple centers published their experience with fresh homograft valve replacements [118,119,120,121,122,123,124,125].
Initial procedures were performed utilizing freshly harvested valves from brain-dead donors or explanted hearts of transplant recipients. Valves were antibiotic-sterilized, stored in nutrient media at 4 °C for up to 6 weeks, and implanted at first opportunity. Such “fresh, wet-stored” homograft valves contained viable cells and were called “homovital” valves. Stanford published on 83 patients who received fresh aortic homografts between 1967 and 1971 and demonstrated a 5-, 10-, and 15-year freedom from valve failure of 83%, 62%, and 43%, respectively. Freedom from reoperation was 88%, 67%, and 45% at 5, 10, and 15 years, respectively [119]. Yacoub’s group also reported their 14-year experience with 275 homovital aortic valve replacements. Freedom from degenerative valve failure was 94% at 5 years and 89% at 10 years [126]. This experience supported that fresh, wet-stored homografts were valuable valve substitutes with good performance data. Donor availability, however, limited widespread use and led to development of current preservation techniques.
In 1975, O’Brien described the cryopreservation of fresh valves using a dimethylsulfoxide controlled-rate freezing technique with storage in liquid nitrogen at − 190 °C [127]. In a series of 192 cryopreserved aortic allografts, the Brisbane group demonstrated 100% freedom from reoperation for valve degeneration at 10 years. Moreover, cryopreserved valves recovered from patients dying of non-valve-related events up to 9.5 years later demonstrated preserved donor fibroblast viability [128]. In 2001, their follow-up series demonstrated that freedom from reoperation for structural deterioration of cryopreserved valves was recipient age-dependent: at 15 years, freedom was 47% (0–20 years), 85% (21–40 years), 81% (41–60 years), and 94% (> 60 years) [129]. This reflects Yacoub’s findings—fresh allograft durability was worse in younger patients, possibly due to recognition of viable cells by a more active immune system.
The degree of cell viability at the time of cryopreserved valve implantation and the rate of cell loss over time is ultimately unknown. It is also unclear whether durability is related to viable donor fibroblasts, improved matrix preservation by cryopreservation, or both. Notably, most early work did not utilize ABO or human leukocyte antigen tissue matching criteria or immunosuppression. Given the overall success with cryopreserved valves and convenience of tissue banking, cryopreservation has become the standard preservation method in modern times. Loss of viability and age-dependent structural degeneration, however, remain the main limitations of cryopreserved homografts.
Delivery of a Living Valve Substitute: Allogeneic Valve Transplantation
There is a critical need for valves with growth potential. Engineering valves capable of growth and regeneration is of great interest, though has failed to achieve clinical translation [130]. Recently, there has been renewed interest in fresh allografts with modifications to preserve viability. Termed “partial heart transplant” or “allogeneic valve transplant,” size-matched donor hearts from a brain-dead donor or explanted hearts of heart transplant recipients are procured [131•]. Semilunar (and possibly atrioventricular valves) are explanted and used to replace diseased recipient valves. This may eliminate the need for anticoagulation and serial reoperations since the allograft should grow with the patient. The major drawback, however, is the presumed need for immunosuppression and its attendant risks.
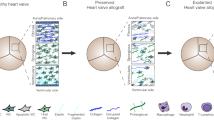
To date, our group and two others have performed a handful of allogeneic valve transplants in infants with truncus arteriosus [6, 7•]; the common truncal root was replaced by donor aortic and pulmonary roots to create two outflows. Recipients are maintained on variable immunosuppression regimens (Fig. 1A), though most are less intense than those used for traditional orthotopic heart transplant. When the child reaches adulthood, the valve may be replaced by a larger prosthesis and immunosuppression discontinued.
Illustrative schematic demonstrating: A presumed role of systemic immunosuppression in preserving valve cell viability and growth potential after allogeneic valve transplant; B donor cardiac allografts deemed non-transplantable and explanted native hearts of transplant recipients—without valvulopathy—may be utilized as a source of fresh valve allografts for allogeneic valve transplantation
Immunologic Considerations of Viable Valve Allografts
Data guiding long-term management are lacking. Some believe that a reduced dose of immunosuppression may be allowable since cardiac valves are less immunogenic than whole cardiac allografts and may even be immune-privileged tissue [131•, 132•]. Mitchell and colleagues examined aortic valves from rejected transplanted hearts and found no evidence of immune injury and entirely preserved cellularity and architecture [133]. Mohri and colleagues orthotopically implanted fresh canine aortic valve allografts into pre-sensitized animals and found no evidence of immune infiltration in the transplanted leaflet at 3 months, but rather signs of re-endothelialization [134]. This concept is further supported by echocardiographic data, which show preserved semilunar valve function despite severe ventricular dysfunction in the setting of acute rejection [135•]. While the mechanism through which valves possess low immunogenicity is unknown, this may be due to the lack of valve tissue vascularity as cellular and antibody-mediated rejection typically occur at the level of the ventricular microvasculature [132•].
Others contend that valves can elicit an immune response [136,137,138,139], with immunogenicity attributed to surface antigens on valve endothelial cells that may attract cytotoxic lymphocytes and induce lymphoproliferation [136]. Hogan and colleagues demonstrated increased recipient T-cell alloreactivity toward donor lymphocytes and persistence of human leukocyte antigen antibodies in patients who received fresh aortic valve allografts [138]. Heslop and colleagues implanted fresh aortic valve allografts into the abdominal aorta of rats and found that immune injury was limited to the rim of myocardium beneath the valve—the aortic wall demonstrated mild antigenicity, but valve leaflets did not show inflammatory changes [139]. Ultimately, additional investigations to determine the extent to which immunosuppression is required to maintain a functional valve are needed.
Future Directions
Though in its infancy, allogeneic valve transplantation is a promising strategy for delivery of a living, viable valve substitute to pediatric patients with irreparable valve disease. For wider incorporation into current practice, safety and efficacy must be further evaluated. The South Carolina group recently proposed a prospective, observational study evaluating safety, feasibility, growth, and function of allogeneic valves [5•]. As these valves are not amenable to biopsy, alternative methods to detect allograft rejection are needed, as are methods to determine the appropriate amount of immunosuppression required for long-term valve function.
We propose that donor hearts declined for conventional transplantation and explanted native hearts of transplant recipients be evaluated for valve donation (Fig. 1B). Additional investigations into valvular cell fate, as well as optimal preservation methods, are also required. Prolonged storage, while maintaining viability, will allow for an extended travel radius for procurement and may have a substantial economic and environmental impact if local procurement teams could obtain and deliver the valve to the recipient hospital by commercial courier services, as is done for kidney transplants.
While the major drawback of allogeneic valve transplant is the presumed need for immunosuppression, methods of inducing graft tolerance under investigation include: (i) thymic co-transplantation, wherein recipient T lymphocytes are educated within the transplanted donor thymus after recipient treatment with T cell–depleting antibodies, and (ii) mixed chimerism, wherein bone marrow-derived cells from both donor and recipient coexist [140, 141]. Most success with these techniques has been in the realm of renal transplantation [142,143,144,145,146], though investigators recently induced donor-specific tolerance with thymic co-transplantation in cardiac allografts for up to 182 days [147]. Finally, advancing our understanding of immune principles in this domain may serve as a platform for future studies in xenogeneic valve transplantation, which could produce a nearly unlimited source of valves [140].
Conclusions
Pediatric valve disease remains a challenging problem in pediatric and congenital cardiac surgery. Currently available valve prostheses lack growth potential, and infants and young children require multiple reoperations. The theoretical growth potential, durability, and lack of anticoagulation of living valve allografts may mitigate the morbidity associated with existing valve replacement options for infants and children. While the major limitation of allogeneic valve transplant is the presumed need for immunosuppression, additional preclinical studies may better reveal the extent to which immunosuppression is required to maintain a functional valve. Still, allogeneic valve transplantation may soon shift the treatment strategy for pediatric valve disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Ely DM, Driscoll AK. Infant mortality in the United States, 2019: data from the period linked birth/infant death file. Natl Vital Stat Rep. 2021;70(14):1–18.
Triedman JK, Newburger JW. Trends in congenital heart disease: the next decade. Circulation. 2016;133(25):2716–33. https://doi.org/10.1161/CIRCULATIONAHA.116.023544.
Simeone RM, Oster ME, Cassell CH, Armour BS, Gray DT, Honein MA. Pediatric inpatient hospital resource use for congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2014;100(12):934–43. https://doi.org/10.1002/bdra.23262.
Henaine R, Roubertie F, Vergnat M, Ninet J. Valve replacement in children: a challenge for a whole life. Arch Cardiovasc Dis. 2012;105(10):517–28. https://doi.org/10.1016/j.acvd.2012.02.013.
Rajab TK, Ochoa B, Zilinskas K, Kwon J, Taylor CL, Henderson HT, Savage AJ, Kavarana M, Turek JW, Costello JM. Partial heart transplantation for pediatric heart valve dysfunction: a clinical trial protocol. PLoS One. 2023;18(2):e0280163. https://doi.org/10.1371/journal.pone.0280163. Comprehensive trial protocol for the clinical evaluation of partial heart transplantation.
Morgan deBlecourt September 09, & deBlecourt, M. (n.d.). Duke Pediatric Heart Surgeons perform world’s first partial heart transplant. Duke Health. Retrieved December 17, 2022, from https://www.dukehealth.org/blog/duke-pediatric-heart-surgeons-perform-worlds-first-partial-heart-transplant.
Rajab TK, Kang L, Hayden K, Andersen ND, Turek JW. New operations for truncus arteriosus repair using partial heart transplantation: exploring the surgical design space with 3-dimensional printed heart models. JTCVS Tech. 2023;13(18):91–6. https://doi.org/10.1016/j.xjtc.2023.02.005. Interesting, novel application of 3D printing to design new operations involving partial heart transplantation.
Brown JW, Stevens LS, Holly S, Robison R, Rodefeld M, Grayson T, Marts B, Caldwell RA, Hurwitz RA, Girod DA, et al. Surgical spectrum of aortic stenosis in children: a thirty-year experience with 257 children. Ann Thorac Surg. 1988;45(4):393–403. https://doi.org/10.1016/s0003-4975(98)90012-1.
Bouhout I, Ba PS, El-Hamamsy I, Poirier N. Aortic valve interventions in pediatric patients. Semin Thorac Cardiovasc Surg. 2019;31:277–87.
Vergnat M, Asfour B, Arenz C, Suchowerskyj P, Bierbach B, Schindler E, et al. Aortic stenosis of the neonate: a single-center experience. J Thorac Cardiovasc Surg. 2019;157:318–26.
Siddiqui J, Brizard CP, Galati JC, Iyengar AJ, Hutchinson D, Konstantinov IE, et al. Surgical valvotomy and repair for neonatal and infant congenital aortic stenosis achieves better results than interventional catheterization. J Am Coll Cardiol. 2013;62:2134–40.
Alsoufi B, Al-Halees Z, Manlhiot C, McCrindle BW, Al-Ahmadi M, Sallehuddin A, Canver CC, Bulbul Z, Joufan M, Fadel B. Mechanical valves versus the Ross procedure for aortic valve replacement in children: propensity-adjusted comparison of long-term outcomes. J Thorac Cardiovasc Surg. 2009;137(2):362-370.e9. https://doi.org/10.1016/j.jtcvs.2008.10.010.
Takkenberg JJ, Kappetein AP, van Herwerden LA, Witsenburg M, Van Osch-Gevers L, Bogers AJ. Pediatric autograft aortic root replacement: a prospective follow-up study. Ann Thorac Surg. 2005;80(5):1628–33. https://doi.org/10.1016/j.athoracsur.2005.04.057.
Rowe G, Gill G, Zubair MM, Roach A, Egorova N, Emerson D, Habib RH, Bowdish ME, Chikwe J, Kim RW. Ross procedure in children: The Society of Thoracic Surgeons Congenital Heart Surgery Database analysis. Ann Thorac Surg. 2023;115(1):119–25. https://doi.org/10.1016/j.athoracsur.2022.06.043. Comprehensive nationwide analysis of the Ross procedure across the pediatric age spectrum.
Moroi MK, Bacha EA, Kalfa DM. The Ross procedure in children: a systematic review. Ann Cardiothorac Surg. 2021;10(4):420–32. https://doi.org/10.21037/acs-2020-rp-23. One the largest recent systematic analyses of the pediatric Ross procedure.
Donald JS, Wallace FRO, Naimo PS, Fricke TA, Brink J, Brizard CP, d’Udekem Y, Konstantinov IE. Ross operation in children: 23-year experience from a single institution. Ann Thorac Surg. 2020;109(4):1251–9. https://doi.org/10.1016/j.athoracsur.2019.10.070.
Martin E, Laurin C, Jacques F, Houde C, Cote JM, Chetaille P, Drolet C, Vaujois L, Kalavrouziotis D, Mohammadi S, Perron J. More than 25 years of experience with the Ross procedure in children: a single-center experience. Ann Thorac Surg. 2020;110(2):638–44. https://doi.org/10.1016/j.athoracsur.2019.10.093.
Nelson JS, Maul TM, Wearden PD, Pasquali SK, Romano JC. National practice patterns and early outcomes of aortic valve replacement in children and teens. Ann Thorac Surg. 2019;108(2):544–51. https://doi.org/10.1016/j.athoracsur.2019.03.098.
Nelson JS, Pasquali SK, Pratt CN, Yu S, Donohue JE, Loccoh E, Ohye RG, Bove EL, Hirsch-Romano JC. Long-term survival and reintervention after the Ross procedure across the pediatric age spectrum. Ann Thorac Surg. 2015;99(6):2086–94. https://doi.org/10.1016/j.athoracsur.2015.02.068. (discussion 2094-5).
Elder RW, Quaegebeur JM, Bacha EA, Chen JM, Bourlon F, Williams IA. Outcomes of the infant Ross procedure for congenital aortic stenosis followed into adolescence. J Thorac Cardiovasc Surg. 2013;145(6):1504–11. https://doi.org/10.1016/j.jtcvs.2012.09.004.
Charitos EI, Takkenberg JJ, Hanke T, Gorski A, Botha C, Franke U, Dodge-Khatami A, Hoerer J, Lange R, Moritz A, Ferrari-Kuehne K, Hetzer R, Huebler M, Bogers AJ, Stierle U, Sievers HH, Hemmer W. Reoperations on the pulmonary autograft and pulmonary homograft after the Ross procedure: an update on the German Dutch Ross Registry. J Thorac Cardiovasc Surg. 2012;144(4):813–21. https://doi.org/10.1016/j.jtcvs.2012.07.005. (discussion 821-3).
Clark JB, Pauliks LB, Rogerson A, Kunselman AR, Myers JL. The Ross operation in children and young adults: a fifteen-year, single-institution experience. Ann Thorac Surg. 2011;91(6):1936–41. https://doi.org/10.1016/j.athoracsur.2010.12.070. (discussion 1941-2).
Hörer J, Stierle U, Bogers AJ, Rein JG, Hetzer R, Sievers HH, Lange R. Re-interventions on the autograft and the homograft after the Ross operation in children. Eur J Cardiothorac Surg. 2010;37(5):1008–14. https://doi.org/10.1016/j.ejcts.2009.10.032.
Takkenberg JJ, Klieverik LM, Schoof PH, van Suylen RJ, van Herwerden LA, Zondervan PE, Roos-Hesselink JW, Eijkemans MJ, Yacoub MH, Bogers AJ. The Ross procedure: a systematic review and meta-analysis. Circulation. 2009;119(2):222–8. https://doi.org/10.1161/CIRCULATIONAHA.107.726349.
Piccardo A, Ghez O, Gariboldi V, Riberi A, Collart F, Kreitmann B, Metras D. Ross and Ross-Konno procedures in infants, children and adolescents: a 13-year experience. J Heart Valve Dis. 2009;18(1):76–82 (discussion 83).
Pasquali SK, Cohen MS, Shera D, Wernovsky G, Spray TL, Marino BS. The relationship between neo-aortic root dilation, insufficiency, and reintervention following the Ross procedure in infants, children, and young adults. J Am Coll Cardiol. 2007;49(17):1806–12. https://doi.org/10.1016/j.jacc.2007.01.071.
Hazekamp MG, Grotenhuis HB, Schoof PH, Rijlaarsdam ME, Ottenkamp J, Dion RA. Results of the Ross operation in a pediatric population. Eur J Cardiothorac Surg. 2005;27(6):975–9. https://doi.org/10.1016/j.ejcts.2005.01.018.
Elkins RC, Lane MM, McCue C. Ross operation in children: late results. J Heart Valve Dis. 2001;10(6):736–41.
Solymar L, Südow G, Holmgren D. Increase in size of the pulmonary autograft after the Ross operation in children: growth or dilation? J Thorac Cardiovasc Surg. 2000;119:4–9.
Charitos EI, Hanke T, Stierle U. Autograft reinforcement to preserve autograft function after the ross procedure: a report from the German-Dutch Ross Registry. Circulation. 2009;120(11 Suppl.):S146–54.
Brown JW, Ruzmetov M, Shahriari AP. Modification of the Ross aortic valve replacement to prevent late autograft dilatation. Eur J Cardiothorac Surg. 2010;37:1002–7.
Bouhout I, Kalfa D, Shah A, Goldstone AB, Harrington J, Bacha E. Surgical management of complex aortic valve disease in young adults: repair, replacement, and future alternatives. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2022;25:28–37. https://doi.org/10.1053/j.pcsu.2022.04.002. Nicely summarizes the current landscape of surgical options for pediatric aortic valve disease.
Husain SA, Brown JW. When reconstruction fails or is not feasible: valve replacement options in the pediatric population. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007;117–24. https://doi.org/10.1053/j.pcsu.2007.01.012.
Myers PO, Mokashi SA, Horgan E, Borisuk M, Mayer JE Jr, Del Nido PJ, Baird CW. Outcomes after mechanical aortic valve replacement in children and young adults with congenital heart disease. J Thorac Cardiovasc Surg. 2019;157(1):329–40. https://doi.org/10.1016/j.jtcvs.2018.08.077.
Etnel JR, Elmont LC, Ertekin E, Mokhles MM, Heuvelman HJ, Roos-Hesselink JW, de Jong PL, Helbing WA, Bogers AJ, Takkenberg JJ. Outcome after aortic valve replacement in children: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2016;151(1):143–52.e1–3. doi: https://doi.org/10.1016/j.jtcvs.2015.09.083.
Karamlou T, Jang K, Williams WG, Caldarone CA, Van Arsdell G, Coles JG, McCrindle BW. Outcomes and associated risk factors for aortic valve replacement in 160 children: a competing-risks analysis. Circulation. 2005;112(22):3462–9. https://doi.org/10.1161/CIRCULATIONAHA.105.541649.
Alexiou C, McDonald A, Langley SM. Aortic valve replacement in children: are mechanical prostheses a good option? Eur J Cardiothorac Surg. 2000;17:125–33.
Champsaur G, Robin J, Tronc F. Mechanical valve in aortic position is a valid option in children and adolescents. Eur J Cardiothorac Surg. 1997;11:117–22.
Saleeb SF, Newburger JW, Geva T, Baird CW, Gauvreau K, Padera RF, Del Nido PJ, Borisuk MJ, Sanders SP, Mayer JE. Accelerated degeneration of a bovine pericardial bioprosthetic aortic valve in children and young adults. Circulation. 2014;130(1):51–60. https://doi.org/10.1161/CIRCULATIONAHA.114.009835.
Mazzitelli D, Guenther T, Schreiber C, Wottke M, Michel J, Meisner H. Aortic valve replacement in children: are we on the right track? Eur J Cardiothorac Surg. 1998;13(5):565–71. https://doi.org/10.1016/s1010-7940(98)00069-4.
Ruzmetov M, Vijay P, Rodefeld MD, Turrentine MW, Brown JW. Evolution of aortic valve replacement in children: a single center experience. Int J Cardiol. 2006;113(2):194–200. https://doi.org/10.1016/j.ijcard.2005.11.011.
Sharabiani MT, Dorobantu DM, Mahani AS, Turner M, Peter Tometzki AJ, Angelini GD, Parry AJ, Caputo M, Stoica SC. Aortic valve replacement and the Ross operation in children and young adults. J Am Coll Cardiol. 2016;67(24):2858–70. https://doi.org/10.1016/j.jacc.2016.04.021.
Xue Y, Kossar AP, Abramov A, Frasca A, Sun M, Zyablitskaya M, Paik D, Kalfa D, Della Barbera M, Thiene G, Kozaki S, Kawashima T, Gorman JH, Gorman RC, Gillespie MJ, Carreon CK, Sanders SP, Levy RJ, Ferrari G. Age-related enhanced degeneration of bioprosthetic valves due to leaflet calcification, tissue crosslinking, and structural changes. Cardiovasc Res. 2023;119(1):302–15. https://doi.org/10.1093/cvr/cvac002.
McClure RS, Narayanasamy N, Wiegerinck E, Lipsitz S, Maloney A, Byrne JG, Aranki SF, Couper GS, Cohn LH. Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: up to 17-year follow-up in 1,000 patients. Ann Thorac Surg. 2010;89(5):1410–6. https://doi.org/10.1016/j.athoracsur.2010.01.046.
Saleeb SF, Gauvreau K, Mayer JE, Newburger JW. Aortic valve replacement with bovine pericardial tissue valve in children and young adults. Circulation. 2019;139(7):983–5. https://doi.org/10.1161/CIRCULATIONAHA.118.037187.
Fukushima S, Tesar PJ, Pearse B, Jalali H, Sparks L, Fraser JF, et al. Long-term clinical outcomes after aortic valve replacement using cryopreserved aortic allograft. J Thorac Cardiovasc Surg. 2014;148:65–72.
Koolbergen DR, Hazekamp MG, de HE, Bruggemans EF, Huysmans HA, Dion RA, et al. The pathology of fresh and cryopreserved homograft heart valves: an analysis of forty explanted homograft valves. J Thorac Cardiovasc Surg. 2002;124:689–97.
Pompilio G, Polvani G, Piccolo G, Guarino A, Nocco A, Innocente A, et al. Six-year monitoring of the donor-specific immune response to cryopreserved aortic allograft valves: implications with valve dysfunction. Ann Thorac Surg. 2004;78:557–63.
Smith JD, Ogino H, Hunt D, Laylor RM, Rose ML, Yacoub MH. Humoral immune response to human aortic valve homografts. Ann Thorac Surg. 1995;60:S127–30.
Sharabiani MT, Dorobantu DM, Mahani AS, Turner M, Peter Tometzki AJ, Angelini GD, et al. Aortic valve replacement and the ross operation in children and young adults. J Am Coll Cardiol. 2016;67:2858–70.
Alsoufi B, Manlhiot C, McCrindle BW. Aortic and mitral valve replacement in children: is there any role for biologic and bioprosthetic substitutes? Eur J Cardiothorac Surg. 2009;36:84–90.
Lupinetti FM, Duncan BW, Lewin M. Comparison of autograft and allograft aortic valve replacement in children. J Thorac Cardiovasc Surg. 2003;126:240–6.
Ozaki S, Kawase I, Yamashita H, Uchida S, Takatoh M, Kiyohara N. Midterm outcomes after aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg. 2018;155:2379–87.
Baird CW, Cooney B, Chávez M, Sleeper LA, Marx GR, Del Nido PJ. Congenital aortic and truncal valve reconstruction using the Ozaki technique: short-term clinical results. J Thorac Cardiovasc Surg. 2021;161(5):1567–77. https://doi.org/10.1016/j.jtcvs.2020.01.087.
Wiggins LM, Mimic B, Issitt R, Ilic S, Bonello B, Marek J, Kostolny M. The utility of aortic valve leaflet reconstruction techniques in children and young adults. J Thorac Cardiovasc Surg. 2020;159(6):2369–78. https://doi.org/10.1016/j.jtcvs.2019.09.176.
Bacha E. Commentary: aortic valve reconstruction with neocuspidization—a word of caution? J Thorac Cardiovasc Surg. 2021;161(5):1578–9. https://doi.org/10.1016/j.jtcvs.2020.02.028.
Nguyen SN, Vinogradsky AV, Sevensky R, Crystal MA, Bacha EA, Goldstone AB. Use of the Inspiris valve in the native right ventricular outflow tract is associated with early prosthetic regurgitation. J Thorac Cardiovasc Surg. 2023;S0022–5223(23):00342–2. https://doi.org/10.1016/j.jtcvs.2023.04.018.
Nomoto R, Sleeper LA, Borisuk MJ, Bergerson L, Pigula FA, Emani S, Fynn-Thompson F, Mayer JE, Del Nido PJ, Baird CW. Outcome and performance of bioprosthetic pulmonary valve replacement in patients with congenital heart disease. J Thorac Cardiovasc Surg. 2016;152(5):1333-1342.e3. https://doi.org/10.1016/j.jtcvs.2016.06.064.
Caldarone CA, McCrindle BW, Van Arsdell GS, Coles JG, Webb G, Freedom RM, Williams WG. Independent factors associated with longevity of prosthetic pulmonary valves and valved conduits. J Thorac Cardiovasc Surg. 2000;120(6):1022–30; discussion 1031. https://doi.org/10.1067/mtc.2000.110684.
Chen PC, Sager MS, Zurakowski D, Pigula FA, Baird CW, Mayer JE Jr, Del Nido PJ, Emani SM. Younger age and valve oversizing are predictors of structural valve deterioration after pulmonary valve replacement in patients with tetralogy of Fallot. J Thorac Cardiovasc Surg. 2012;143(2):352–60. https://doi.org/10.1016/j.jtcvs.2011.10.079.
Pragt H, van Melle JP, Javadikasgari H, Seo DM, Stulak JM, Knez I, Hörer J, Muñoz-Guijosa C, Dehaki MG, Shin HJ, Dearani JA, Dehaki MG, Pieper PG, Eulenburg C, Dos L, Ebels T. Mechanical valves in the pulmonary position: an international retrospective analysis. J Thorac Cardiovasc Surg. 2017;154(4):1371-1378.e1. https://doi.org/10.1016/j.jtcvs.2017.04.072.
Freling HG, van Slooten YJ, van Melle JP, Ebels T, Hoendermis ES, Berger RM, Hillege HL, Waterbolk TW, van Veldhuisen DJ, Willems TP, Pieper PG. Pulmonary valve replacement: twenty-six years of experience with mechanical valvar prostheses. Ann Thorac Surg. 2015;99(3):905–10. https://doi.org/10.1016/j.athoracsur.2014.10.034.
Dunne B, Xiao A, Litton E, Andrews D. Mechanical prostheses for right ventricular outflow tract reconstruction: a systematic review and meta-analysis. Ann Thorac Surg. 2015;99(5):1841–7. https://doi.org/10.1016/j.athoracsur.2014.11.058.
Shin HJ, Kim YH, Ko JK, Park IS, Seo DM. Outcomes of mechanical valves in the pulmonic position in patients with congenital heart disease over a 20-year period. Ann Thorac Surg. 2013;95(4):1367–71. https://doi.org/10.1016/j.athoracsur.2012.07.008.
Stulak JM, Dearani JA, Burkhart HM, Connolly HM, Warnes CA, Suri RM, Schaff HV. The increasing use of mechanical pulmonary valve replacement over a 40-year period. Ann Thorac Surg. 2010;90(6):2009–14; discussion 2014–5. https://doi.org/10.1016/j.athoracsur.2010.07.023.
Haas F, Schreiber C, Hörer J, Kostolny M, Holper K, Lange R. Is there a role for mechanical valved conduits in the pulmonary position? Ann Thorac Surg. 2005;79(5):1662–7; discussion 1667–8. https://doi.org/10.1016/j.athoracsur.2004.10.054.
Law MA, Chatterjee A. Transcatheter pulmonic valve implantation: techniques, current roles, and future implications. World J Cardiol. 2021;13(5):117–29. https://doi.org/10.4330/wjc.v13.i5.117.
Jones TK, McElhinney DB, Vincent JA, Hellenbrand WE, Cheatham JP, Berman DP, Zahn EM, Khan DM, Rhodes JF Jr, Weng S, Bergersen LJ. Long-term outcomes after Melody transcatheter pulmonary valve replacement in the US investigational device exemption trial. Circ Cardiovasc Interv. 2022;15(1):e010852. https://doi.org/10.1161/CIRCINTERVENTIONS.121.010852.
Ribeiro JM, Teixeira R, Lopes J, Costa M, Pires A, Gonçalves L. Transcatheter versus surgical pulmonary valve replacement: a systemic review and meta-analysis. Ann Thorac Surg. 2020;110(5):1751–61. https://doi.org/10.1016/j.athoracsur.2020.03.007.
Cheatham JP, Hellenbrand WE, Zahn EM, Jones TK, Berman DP, Vincent JA, McElhinney DB. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US Melody valve investigational device exemption trial. Circulation. 2015;131(22):1960–70. https://doi.org/10.1161/CIRCULATIONAHA.114.013588.
Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter pulmonary valve replacement with the Edwards Sapien system: the Toronto experience. JACC Cardiovasc Interv. 2015;8(14):1819–27. https://doi.org/10.1016/j.jcin.2015.08.016.
Dearani JA, Danielson GK, Puga FJ, Schaff HV, Warnes CW, Driscoll DJ, Schleck CD, Ilstrup DM. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg. 2003;75(2):399–410; discussion 410–1. https://doi.org/10.1016/s0003-4975(02)04547-2.
Lewis MJ, Malm T, Hallbergson A, Nilsson F, Ramgren JJ, Tran K, Liuba P. Long-term follow-up of right ventricle to pulmonary artery biologic valved conduits used in pediatric congenital heart surgery. Pediatr Cardiol. 2023;44(1):102–15. https://doi.org/10.1007/s00246-022-02956-3.
Havova M, Gebauer R, Antonova P, Spatenka J, Burkert J, Fabian O, Modrak M, Rohn V. Clinical experience of reoperative right ventricular outflow tract reconstruction with valved conduits: risk factors for conduit failure in long-term follow-up. Cell Tissue Bank. 2023. https://doi.org/10.1007/s10561-023-10088-y.
Boethig D, Goerler H, Westhoff-Bleck M, Ono M, Daiber A, Haverich A, Breymann T. Evaluation of 188 consecutive homografts implanted in pulmonary position after 20 years. Eur J Cardiothorac Surg. 2007;32(1):133–42. https://doi.org/10.1016/j.ejcts.2007.02.025.
Meyns B, Jashari R, Gewillig M, Mertens L, Komárek A, Lesaffre E, Budts W, Daenen W. Factors influencing the survival of cryopreserved homografts. The second homograft performs as well as the first. Eur J Cardiothorac Surg. 2005;28(2):211–6; discussion 216. https://doi.org/10.1016/j.ejcts.2005.03.041.
Tweddell JS, Pelech AN, Frommelt PC, Mussatto KA, Wyman JD, Fedderly RT, Berger S, Frommelt MA, Lewis DA, Friedberg DZ, Thomas JP Jr, Sachdeva R, Litwin SB. Factors affecting longevity of homograft valves used in right ventricular outflow tract reconstruction for congenital heart disease. Circulation. 2000;102(19 Suppl 3):III130–5. doi: https://doi.org/10.1161/01.cir.102.suppl_3.iii-130.
Homann M, Haehnel JC, Mendler N, Paek SU, Holper K, Meisner H, Lange R. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur J Cardiothorac Surg. 2000;17(6):624–30. https://doi.org/10.1016/s1010-7940(00)00414-0.
Niwaya K, Knott-Craig CJ, Lane MM, Chandrasekaren K, Overholt ED, Elkins RC. Cryopreserved homograft valves in the pulmonary position: risk analysis for intermediate-term failure. J Thorac Cardiovasc Surg. 1999;117(1):141–6; discussion 46–7. https://doi.org/10.1016/s0022-5223(99)70479-4.
LeBlanc JG, Russell JL, Sett SS, Potts JE. Intermediate follow-up of right ventricular outflow tract reconstruction with allograft conduits. Ann Thorac Surg. 1998;66(6 Suppl):S174–8. https://doi.org/10.1016/s0003-4975(98)01032-7.
Brown JW, Ruzmetov M, Rodefeld MD, Vijay P, Turrentine MW. Right ventricular outflow tract reconstruction with an allograft conduit in non-Ross patients: risk factors for allograft dysfunction and failure. Ann Thorac Surg. 2005;80(2):655–63; discussion 663–4. https://doi.org/10.1016/j.athoracsur.2005.02.053.
Askovich B, Hawkins JA, Sower CT, Minich LL, Tani LY, Stoddard G, Puchalski MD. Right ventricle-to-pulmonary artery conduit longevity: is it related to allograft size? Ann Thorac Surg. 2007;84(3):907–11; discussion 911–2. https://doi.org/10.1016/j.athoracsur.2007.04.104.
Levine JC, Mayer JE Jr, Keane JF, Spevak PJ, Sanders SP. Anastomotic pseudoaneurysm of the ventricle after homograft placement in children. Ann Thorac Surg. 1995;59(1):60–6. https://doi.org/10.1016/0003-4975(94)00569-S.
Emani SM. Options for prosthetic pulmonary valve replacement. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15(1):34–7. https://doi.org/10.1053/j.pcsu.2012.01.015.
Morray BH, McElhinney DB, Boudjemline Y, Gewillig M, Kim DW, Grant EK, Bocks ML, Martin MH, Armstrong AK, Berman D, Danon S, Hoyer M, Delaney JW, Justino H, Qureshi AM, Meadows JJ, Jones TK. Multicenter experience evaluating transcatheter pulmonary valve replacement in bovine jugular vein (Contegra) right ventricle to pulmonary artery conduits. Circ Cardiovasc Interv. 2017;10(6):e004914. https://doi.org/10.1161/CIRCINTERVENTIONS.116.004914.
Mery CM, Guzmán-Pruneda FA, De León LE, Zhang W, Terwelp MD, Bocchini CE, Adachi I, Heinle JS, McKenzie ED, Fraser CD Jr. Risk factors for development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. J Thorac Cardiovasc Surg. 2016;151(2):432–9, 441.e1–2. https://doi.org/10.1016/j.jtcvs.2015.10.069.
Brown JW, Ruzmetov M, Rodefeld MD, Eltayeb O, Yurdakok O, Turrentine MW. Contegra versus pulmonary homografts for right ventricular outflow tract reconstruction: a ten-year single-institution comparison. World J Pediatr Congenit Heart Surg. 2011;2(4):541–9. https://doi.org/10.1177/2150135111415711.
Hickey EJ, McCrindle BW, Blackstone EH, Yeh T Jr, Pigula F, Clarke D, et al. Jugular venous valved conduit (Contegra) matches allograft performance in infant truncus arteriosus repair. Eur J Cardiothorac Surg. 2008;33:890–8. https://doi.org/10.1016/j.ejcts.2007.12.052.
Sekarski N, van Meir H, Rijlaarsdam ME, Schoof PH, Koolbergen DR, Hruda J, von Segesser LK, Meijboom EJ, Hazekamp MG. Right ventricular outflow tract reconstruction with the bovine jugular vein graft: 5 years’ experience with 133 patients. Ann Thorac Surg. 2007;84(2):599–605. https://doi.org/10.1016/j.athoracsur.2007.04.026.
Poynter JA, Eghtesady P, McCrindle BW, Walters HL 3rd, Kirshbom PM, Blackstone EH, et al. Congenital heart surgeons: association of pulmonary conduit type and size with durability in infants and young children. Ann Thorac Surg. 2013;96:1695–701; discussion 1701–2. https://doi.org/10.1016/j.athoracsur.2013.05.074
Ando M, Takahashi Y. Ten-year experience with handmade trileaflet polytetrafluoroethylene valved conduit used for pulmonary reconstruction. J Thorac Cardiovasc Surg. 2009;137(1):124–31. https://doi.org/10.1016/j.jtcvs.2008.08.060.
Belli E, Salihoğlu E, Leobon B, Roubertie F, Ly M, Roussin R, Serraf A. The performance of Hancock porcine-valved Dacron conduit for right ventricular outflow tract reconstruction. Ann Thorac Surg. 2010;89(1):152–7; discussion 157–8. https://doi.org/10.1016/j.athoracsur.2009.09.046.
Brown JW, Fiore AC, Ruzmetov M, Eltayeb O, Rodefeld MD, Turrentine MW. Evolution of mitral valve replacement in children: a 40-year experience. Ann Thorac Surg. 2012;93:626–33.
Caldarone CA, Raghuveer G, Hills CB, Atkins DL, Burns TL, Behrendt DM, Moller JH. Long-term survival after mitral valve replacement in children aged <5 years: a multi-institutional study. Circulation. 2001;104(12 Suppl 1):I143–7. https://doi.org/10.1161/hc37t1.094840.
van Doorn C, Yates R, Tsang V, deLeval M, Elliott M. Mitral valve replacement in children: mortality, morbidity, and haemodynamic status up to medium term follow up. Heart. 2000;84(6):636–42. https://doi.org/10.1136/heart.84.6.636.
Alexiou C, Galogavrou M, Chen Q, McDonald A, Salmon AP, Keeton BK, Haw MP, Monro JL. Mitral valve replacement with mechanical prostheses in children: improved operative risk and survival. Eur J Cardiothorac Surg. 2001;20(1):105–13. https://doi.org/10.1016/s1010-7940(01)00763-1.
Ibezim C, Sarvestani AL, Knight JH, Qayum O, Alshami N, Turk E, St Louis J, McCracken C, Moller JH, Kochilas L, Raghuveer G. Outcomes of mechanical mitral valve replacement in children. Ann Thorac Surg. 2019;107(1):143–50. https://doi.org/10.1016/j.athoracsur.2018.07.069.
Eble BK, Fiser WP, Simpson P, Dugan J, Drummond-Webb JJ, Yetman AT. Mitral valve replacement in children: predictors of long-term outcome. Ann Thorac Surg. 2003;76:853–9.
Kojori F, Chen R, Caldarone CA, Merklinger SL, Azakie A, Williams WG, et al. Outcomes of mitral valve replacement in children: a competing-risks analysis. J Thorac Cardiovasc Surg. 2004;128:703–9.
Selamet Tierney ES, Pigula FA, Berul CI, Lock JE, del Nido PJ, McElhinney DB. Mitral valve replacement in infants and children 5 years of age or younger: evolution in practice and outcome over three decades with a focus on supra-annular prosthesis implantation. J Thorac Cardiovasc Surg. 2008;136:954-61.e3.
Choi PS, Sleeper LA, Lu M, Upchurch P, Baird C, Emani SM. Revisiting prosthesis choice in mitral valve replacement in children: durable alternatives to traditional bioprostheses. J Thorac Cardiovasc Surg. 2020;S0022–5223(20):31281–2. https://doi.org/10.1016/j.jtcvs.2020.04.173. Spans nearly three decades and reveals key risk factors for mitral prosthesis failure.
Freud LR, Marx GR, Marshall AC, Tworetzky W, Emani SM. Assessment of the Melody valve in the mitral position in young children by echocardiography. J Thorac Cardiovasc Surg. 2017;153(1):153-160.e1. https://doi.org/10.1016/j.jtcvs.2016.07.017.
Quinonez LG, Breitbart R, Tworetsky W, Lock JE, Marshall AC, Emani SM. Stented bovine jugular vein graft (Melody valve) for surgical mitral valve replacement in infants and children. J Thorac Cardiovasc Surg. 2013;148:1443.
Dranseika V, Pretre R, Kretschmar O, Dave H. Melody valve to replace the mitral valve in small children: lessons learned. Ann Pediatr Cardiol. 2021;14(1):35–41. https://doi.org/10.4103/apc.APC_74_20.
Pluchinotta FR, Piekarski BL, Milani V, Kretschmar O, Burch PT, Hakami L, Meyer DB, Jacques F, Ghez O, Trezzi M, Carotti A, Qureshi SA, Michel-Behnke I, Hammel JM, Chai P, McMullan D, Mettler B, Ferrer Q, Carminati M, Emani SM. Surgical atrioventricular valve replacement with Melody valve in infants and children. Circ Cardiovasc Interv. 2018;11(11):e007145. https://doi.org/10.1161/CIRCINTERVENTIONS.118.007145.
Robbins RC, Bowman FO Jr, Malm JR. Cardiac valve replacement in children: a twenty-year series. Ann Thorac Surg. 1988;45(1):56–61. https://doi.org/10.1016/s0003-4975(10)62398-3.
Bartlett HL, Atkins DL, Burns TL, Engelkes KJ, Powell SJ, Hills CB, Moller JH. Early outcomes of tricuspid valve replacement in young children. Circulation. 2007;115(3):319–25. https://doi.org/10.1161/CIRCULATIONAHA.106.618652.
Kawahira Y, Yagihara T, Uemura H, Yoshizumi K, Yoshikawa Y, Kitamura S. Replacement of the tricuspid valve in children with congenital cardiac malformations. J Heart Valve Dis. 2000;9(5):636–40.
Kiziltan HT, Theodoro DA, Warnes CA, O’Leary PW, Anderson BJ, Danielson GK. Late results of bioprosthetic tricuspid valve replacement in Ebstein’s anomaly. Ann Thorac Surg. 1998;66(5):1539–45. https://doi.org/10.1016/s0003-4975(98)00961-8.
Kaplan M, Kut MS, Demirtas MM, Cimen S, Ozler A. Prosthetic replacement of tricuspid valve: bioprosthetic or mechanical. Ann Thorac Surg. 2002;73(2):467–73. https://doi.org/10.1016/s0003-4975(01)03128-9.
Nakano K, Eishi K, Kosakai Y, Isobe F, Sasako Y, Nagata S, Ueda H, Kito Y, Kawashima Y. Ten-year experience with the Carpentier-Edwards pericardial xenograft in the tricuspid position. J Thorac Cardiovasc Surg. 1996;111(3):605–12. https://doi.org/10.1016/s0022-5223(96)70312-4.
Rizzoli G, Vendramin I, Nesseris G, Bottio T, Guglielmi C, Schiavon L. Biological or mechanical prostheses in tricuspid position? A meta-analysis of intra-institutional results. Ann Thorac Surg. 2004;77(5):1607–14. https://doi.org/10.1016/j.athoracsur.2003.10.015.
Boyd R, Kalfa D, Nguyen S, Setton M, Shah A, Karamichalis J, Lewis M, Wassercug NZ, Rosenbaum M, Bacha E. Comparative outcomes and risk analysis after cone repair or tricuspid valve replacement for Ebstein’s anomaly. JTCVS Open. 2023. https://doi.org/10.1016/j.xjon.2023.03.004.
Garatti A, Nano G, Bruschi G, et al. Twenty-five year outcomes of tricuspid valve replacement comparing mechanical and biologic prostheses. Ann Thorac Surg. 2012;93:1146–53.
Ross DN. Homograft replacement of the aortic valve. Lancet. 1962;2:487.
Barratt-Boyes BG. Homograft aortic valve replacement and aortic incompetence and stenosis. Thorax. 1964;19(2):131–50. https://doi.org/10.1136/thx.19.2.131.
Barratt-Boyes B, Boche A, Agnew TM, Cole D, Kerr A, Monro JL, et al. Homograft valves. Med J Aust. 1972;2:38–44.
Anderson ET, Hancock EW. Long-term follow-up of aortic valve replacement with the fresh aortic homograft. J Thorac Cardiovasc Surg. 1976;72(1):150–6.
Miller DC, Shumway NE. “Fresh” aortic allografts: long-term results with free-hand aortic valve replacement. J Card Surg. 1987;2(1 Suppl):185–91. https://doi.org/10.1111/jocs.1987.2.1s.185.
Thompson R, Knight E, Ahmed M, Somerville W, Towers M, Yacoub M. The use of “fresh” unstented homograft valves for replacement of the aortic valve: analysis of 6 1/2 years experience. Circulation. 1977;56(5):837–41. https://doi.org/10.1161/01.cir.56.5.837.
Penta A, Qureshi S, Radley-Smith R, Yacoub MH. Patient status 10 or more years after ‘fresh’ homograft replacement of the aortic valve. Circulation. 1984;70(3 Pt 2):I182–6.
Thompson R, Yacoub M, Ahmed M, Seabra-Gomes R, Rickards A, Towers M. Influence of preoperative left ventricular function on results of homograft replacement of the aortic valve for aortic stenosis. Am J Cardiol. 1979;43(5):929–38. https://doi.org/10.1016/0002-9149(79)90355-2.
Thompson R, Ahmed M, Seabra-Gomes R, Ilsley C, Rickards A, Towers M, Yacoub M. Influence of preoperative left ventricular function on results of homograft replacement of the aortic valve for aortic regurgitation. J Thorac Cardiovasc Surg. 1979;77(3):411–21.
Khanna SK, Ross JK, Monro JL. Homograft aortic valve replacement: seven years’ experience with antibiotic-treated valves. Thorax. 1981;36(5):330–7. https://doi.org/10.1136/thx.36.5.330.
Barratt-Boyes BG, Roche AH, Subramanyan R, Pemberton JR, Whitlock RM. Long-term follow-up of patients with the antibiotic-sterilized aortic homograft valve inserted freehand in the aortic position. Circulation. 1987;75(4):768–77. https://doi.org/10.1161/01.cir.75.4.768.
Yacoub M, Rasmi NR, Sundt TM, Lund O, Boyland E, Radley-Smith R, Khaghani A, Mitchell A. Fourteen-year experience with homovital homografts for aortic valve replacement. J Thorac Cardiovasc Surg. 1995;110(1):186–93; discussion 193–4. https://doi.org/10.1016/S0022-5223(05)80025-X.
O’Brien MF, Stafford G, Gardner M, Pohlner P, McGiffin D, Johnston N, Brosnan A, Duffy P. The viable cryopreserved allograft aortic valve. J Card Surg. 1987;2(1 Suppl):153–67. https://doi.org/10.1111/jocs.1987.2.1s.153.
O’Brien MF, Stafford EG, Gardner MA, Pohlner PG, McGiffin DC. A comparison of aortic valve replacement with viable cryopreserved and fresh allograft valves, with a note on chromosomal studies. J Thorac Cardiovasc Surg. 1987;94(6):812–23.
O’Brien MF, Harrocks S, Stafford EG, Gardner MA, Pohlner PG, Tesar PJ, Stephens F. The homograft aortic valve: a 29-year, 99.3% follow up of 1,022 valve replacements. J Heart Valve Dis. 2001;10(3):334–44; discussion 335.
Boyd R, Parisi F, Kalfa D. State of the art: tissue engineering in congenital heart surgery. Semin Thorac Cardiovasc Surg. 2019 Winter;31(4):807–817. https://doi.org/10.1053/j.semtcvs.2019.05.023.
Rajab TK. Evidence-based surgical hypothesis: partial heart transplantation can deliver growing valve implants for congenital cardiac surgery. Surgery. 2021;169(4):983–5. https://doi.org/10.1016/j.surg.2020.07.051. Thought-provoking piece on the growth potential of heart valve transplants with limited or no need for immunosuppression.
Hill MA, Kwon JH, Gerry B, Hardy WA, Walkowiak OA, Kavarana MN, Nadig SN, Rajab TK. Immune privilege of heart valves. Front Immunol. 2021;10(12):731361. https://doi.org/10.3389/fimmu.2021.731361. Early work presenting the idea of heart valves as immune-privileged sites.
Mitchell RN, Jonas RA, Schoen FJ. Pathology of explanted cryopreserved allograft heart valves: comparison with aortic valves from orthotopic heart transplants. J Thorac Cardiovasc Surg. 1998;115(1):118–27. https://doi.org/10.1016/s0022-5223(98)70450-7.
Mohri H, Reichenbach DD, Barnes RW, Nelson RJ, Merendino KA. Studies of antigenicity of the homologous aortic valve. J Thorac Cardiovasc Surg. 1967;54(4):564–72.
McVadon DH, Hardy WA, Boucek KA, Rivers WD, Kwon JH, Kavarana MN, Costello JM, Rajab TK. Effect of cardiac graft rejection on semilunar valve function: implications for heart valve transplantation. Cardiol Young. 2022;15:1–8. https://doi.org/10.1017/S104795112200258X. Small study demonstrating preserved valve function during rejection; supports notion that valve transplant recipients may require reduced immunosuppression.
Batten P, McCormack AM, Rose ML, Yacoub MH. Valve interstitial cells induce donor-specific T-cell anergy. J Thorac Cardiovasc Surgerya. 2001;122(1):129–35. https://doi.org/10.1067/mtc.2001.114940.
Hoekstra F, Knoop C, Aghai Z, Jutte N, Mochtar B, Bos E, Weimar W. Stimulation of immune-competent cells in vitro by human cardiac valve-derived endothelial cells. Ann Thorac Surg. 1995;60(2 Suppl):S131–3; discussion S133–4. https://doi.org/10.1016/0003-4975(95)00273-n.
Hogan P, Duplock L, Green M, Smith S, Gall KL, Frazer IH, O’Brien MF. Human aortic valve allografts elicit a donor-specific immune response. J Thorac Cardiovasc Surg. 1996;112(5):1260–6; discussion 1266–7. https://doi.org/10.1016/S0022-5223(96)70139-3.
Heslop BF, Wilson SE, Hardy BE. Antigenicity of aortic valve allografts. Ann Surg. 1973;177(3):301–6. https://doi.org/10.1097/00000658-197303000-00010.
Goldstone AB, Bacha EA, Sykes M. On cardiac xenotransplantation and the role of xenogeneic tolerance. J Thorac Cardiovasc Surg. 2022:S0022–5223(22)01340-X. https://doi.org/10.1016/j.jtcvs.2022.11.036.
Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med. 2014;4(1):a015529. https://doi.org/10.1101/cshperspect.a015529.
Yamada K, Shimizu A, Ierino FL, Utsugi R, Barth RN, Esnaola N, Colvin RB, Sachs DH. Thymic transplantation in miniature swine. I. Development and function of the “thymokidney.” Transplantation. 1999;68(11):1684–92. https://doi.org/10.1097/00007890-199912150-00011.
Yamada K, Shimizu A, Utsugi R, Ierino FL, Gargollo P, Haller GW, et al. Thymic transplantation in miniature swine, II: induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol. 2000;164:3079–86.
Yamada K, Vagefi PA, Utsugi R, Kitamura H, Barth RN, LaMattina JC, et al. Thymic transplantation in miniature swine, III: induction of tolerance by transplantation of composite thymokidneys across fully major histocompatibility complex mismatched barriers. Transplantation. 2003;76:530–6.
Yamada K, Gianello PR, Ierino FL, Lorf T, Shimizu A, Meehan S, Colvin RB, Sachs DH. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186(4):497–506. https://doi.org/10.1084/jem.186.4.497.
Kamano C, Vagefi PA, Kumagai N, Yamamoto S, Barth RN, LaMattina JC, Moran SG, Sachs DH, Yamada K. Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci U S A. 2004;101(11):3827–32. https://doi.org/10.1073/pnas.0306666101.
Nobori S, Samelson-Jones E, Shimizu A, Hisashi Y, Yamamoto S, Kamano C, et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation. 2006;8:26–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
G.F. is partially supported by NIH R01 HL 163085.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, S.N., Vinogradsky, A.V., Ferrari, G. et al. Pitfalls and Future Directions of Contemporary Pediatric Valve Surgery: the Case for Living Valve Substitutes. Curr Pediatr Rep 11, 180–192 (2023). https://doi.org/10.1007/s40124-023-00295-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40124-023-00295-2