Abstract
Introduction
To analyze the efficacy and safety profile of the intravitreal ranibizumab biosimilar molecule, Razumab® (Intas Pharmaceuticals, Ahmedabad, India; BRm; Razumab®) and the innovator ranibizumab drug (IRm; LUCENTIS®) in Indian patients with polypoidal choroidal vasculopathy (PCV) under real-world conditions.
Methods
This was a retrospective study of treatment-naïve and previously treated PCV eyes undergoing intravitreal therapy with either BRm or IRm from January 2019 to September 2020 as three loading doses followed by a pro-re-nata (PRN) regimen. Changes in the best-corrected visual acuity (BCVA), subretinal fluid (SRF), intraretinal fluid (IRF), SRF height, and subfoveal choroidal thickness (SFCT) and the safety profiles were assessed at weeks 12, 24, and 52, respectively.
Results
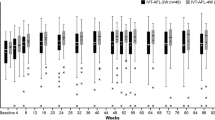
A total of 22 eyes received IRm and 19 eyes underwent BRm therapy, respectively. Both the groups were comparable in age (P = 0.41) and gender distribution, although the BRm arm had significantly more eyes that were previously treated (P < 0.00001) with a greater median number of injections (P < 0.0001). At week 52, both groups had similar gains in visual acuity (P = 0.19), SRF resolution (P = 0.8), IRF resolution (P = 0.47), and SRF height (P = 0.71). The IRm eyes exhibited a significant improvement in BCVA (P = 0.001) at all visits with a greater mean number of injections (IRm: 5.41 ± 0.94; BRm: 4 ± 1.45; P = 0.0004), while the BRm eyes showed a similar increase in BCVA but did not reach statistical significance until week 52. The SFCT decreased significantly in the BRm arm at week 52 (P = 0.045). One eye (5.26%) in the BRm arm experienced mild anterior uveitis, which was treated with topical corticosteroids. In either arm, no other ocular or systemic adverse effects were observed.
Conclusions
Our real-world data demonstrated the ranibizumab biosimilar Razumab to have comparable visual acuity outcomes to the innovator ranibizumab molecule with an adequate safety profile in the management of PCV. Although these encouraging results support its use as a viable alternative to the innovator molecule, further prospective studies in a diverse patient population are needed to validate our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Anti-vascular endothelial growth factor (anti-VEGF) biosimilars are an alluring therapeutic alternative to the original biologic because they have the ability to lower the overall therapeutic cost in the management of varied chorioretinal disorders. |
Razumab® (Intas Pharmaceuticals, Ahmedabad, India) is an Indian regulatory-approved ranibizumab biosimilar that has shown encouraging results in the management of neovascular age-related macular degeneration (n-AMD), retinal vein occlusion (RVO), and diabetic macular edema (DME). |
The real-world outcomes of the biosimilar ranibizumab molecule (Razumab®) therapy in polypoidal choroidal vasculopathy (PCV) have not been adequately described and their direct comparison with the innovator ranibizumab molecule (IRm; LUCENTIS®) remains unreported. |
What was learned from the study? |
In patients with treatment-naive and previously treated PCV, real-world 52-week therapy data shows that the innovator-ranibizumab drug (LUCENTIS®) and the biosimilar-ranibizumab drug(RAZUMAB®) are similarly effective at increasing visual acuity with comparable fluid resolution (subretinal and intraretinal) and an acceptable safety profile. |
Razumab®, a regulatory approved ranibizumab biosimilar, is an effective and safe alternative to the branded drug that has the potential to improve the health economics of PCV treatment. |
Introduction
Polypoidal choroidal vasculopathy (PCV) was initially reported by Yannuzzi et al. [1], which is characterized by choroidal abnormality with a high prevalence in Asian populations [2]. It forms a part of the pachychoroid disease spectrum, which is phenotypically characterized by increased choroidal thickness, with/without the presence of pachyvessels, and marked attenuation of choriocapillaris [2]. PCV is characterized by an abnormal branching vascular network (BVN) and polypoidal dilations noted on indocyanine green angiography (ICGA), which is the gold standard for diagnosing PCV [3]. PCV accounts for 10–54% of previously diagnosed cases of neovascular age-related macular degeneration (nAMD) cases in Asian populations [4, 5]. PCV is characterized by recurrent episodes of hemorrhage and exudative maculopathy, which may cause significant and permanent vision loss and therefore seriously affect the quality of life [6]. Various therapeutic options for PCV include intravitreal anti-vascular endothelial growth factor (VEGF) agents, verteporfin photodynamic therapy (PDT), and combined therapy with anti-VEGF and PDT.
Ranibizumab is a humanized monoclonal antibody that binds with VEGF-A isoforms and prevents its interaction with its receptors on endothelial cells surface [7]. The use of the innovator ranibizumab molecule (IRm; LUCENTIS®; Genentech, S. San Francisco, CA/Roche, Basel, Switzerland) monotherapy to treat PCV have been explored in the EVEREST and the LAPTOP clinical trials, which reported that ranibizumab was more effective in improving VA than PDT [7, 8]. Various other studies have proved vision improvement with anti-VEGF monotherapy [9,10,11].
Biosimilars agents have almost the same structural and functional properties as parent biological molecule. These drugs could be a significant step forward in the treatment of retinal diseases. Razumab® (Intas Pharmaceuticals, Ahmedabad, India) is the world’s first biosimilar of ranibizumab (BRm) which was approved by the Drug Controller General of India in 2015 for its usage in the management of nAMD and macular edema secondary to retinal vein occlusions. The regulatory approval was based upon the encouraging results from the RE-ENACT and RE-ENACT two trials which indicated that Razumab efficiently treats eyes with nAMD and macular edema caused by retinal vein occlusions (RVO) [12, 13].
The real-world outcomes of BRm therapy in PCV are yet to be extensively reported, and no study has directly compared BRm to IRm in PCV. In the current study, we report the efficacy and safety profile of BRm in PCV and compare its treatment outcomes with that of IRm.
Methods
This was a retrospective chart review of treatment-naïve and previously treated eyes with PCV patients undergoing treatment with either IRm or BRm from January 2019 to September 2020 at Chaithanya Eye Hospital, Trivandrum, India. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics committee of Chaithanya Eye Hospital, Trivandrum, India. Written informed consent for treatment and data collection was obtained from each patient.
Patient Recruitment and Treatment
Forty-one patients with an ICGA diagnosed PCV were advised treatment with either IRm or BRm injection. Patients were excluded if they had concomitant ophthalmology conditions such as media opacities which limited the ability to acquire good images or if they had other retinal pathology such as macular neovascularization (MNV) due to AMD or other etiologies. Patients underwent three loading doses of intravitreal anti-VEGF injections (BRm or IRm; 0.5 mg in 0.05 ml) followed by a pro-re-nata (PRN) regimen. All injections were performed in an operating theater (OT) under a strict aseptic technique. The patients returned for monthly follow-up visits for 1 year. At each visit, the patients underwent a comprehensive eye examination including slit-lamp biomicroscopy, tonometry, fundus examination with indirect ophthalmoscopy, and spectral-domain OCT (SD-OCT) analysis. Fundus fluorescein angiography (FFA) and ICGA were performed at baseline and repeated in cases that were non-responsive after the three loading doses or in recalcitrant cases.
The patients were divided into two groups based upon the anti-VEGF agent: Group 1: IRm and Group 2: BRm. The primary endpoint of the study were the changes in the best-corrected visual acuity (BCVA), subretinal fluid (SRF), intraretinal fluid (IRF), subfoveal choroidal thickness (SFCT), and a safety analysis at weeks 12, 24, and 52. Retreatment was guided by disease activity evident with new or persistent IRF/SRF on SD-OCT or appearance of a new hemorrhage clinically.
Statistical Analysis
Statistical analysis was performed by SPSS 23.0 version. Continuous variables were described as mean and variation of each observation from the mean value (standard deviation) represented as mean ± SD. Continuous variables following normal distribution were analyzed using unpaired t test whereas those not following normal distribution were analyzed using the Mann–Whitney U test. Paired variables were analyzed using paired t test (for normal distribution) and Wilcoxon signed-rank test (for non-normal distribution). Categorical variables were described by taking percentages and were analyzed using the Chi-square test or Fisher’s exact test when appropriate. Paired categorical variables were analyzed using the McNemar test. Variables with p value < 0.05 were considered statistically significant.
Results
Study Population
Forty-one eyes of 41 patients with PCV were included in the study. Of these, 21 eyes underwent IRm therapy and 19 eyes received BRm. There was no significant difference between the two groups in relation to age (IRm: 67.27 ± 8.14 years; BRm: 69.53 ± 9.12 years; P = 0.41) and gender distribution. A significantly greater number of eyes in the IRm arm were treatment-naïve as compared to the BRm arm (IRm: 19/22; BRm: 2/19; P < 0.00001). The median number of anti-VEGF injections received prior to switching to BRm was significantly higher as compared in the IRm arm (BRm: 5 [range 0–20]; IRm: 0 [range 0–5]; P < 0.001). Table 1 lists the comparison of demographic and tomographic features between the two groups at baseline.
Visual Acuity
At baseline, there was no significant difference in the median logMAR BCVA between the two groups (IRm: 0.47 [range 0.27–0.5]; BRm: 0.48 [range 0.18–0.6]; P = 0.98). After undergoing intravitreal ranibizumab therapy, no significant difference in BCVA was seen at weeks 12, 24, and 52 between the two arms. The IRm eyes demonstrated a significant improvement in BCVA at all visits, whereas the BRm eyes showed an improvement in BCVA but did not achieve statistical significance until week 52.
SD-OCT Analysis
No significant difference was noted in the proportion of eyes having SRF at baseline and up to week 52 between the two groups. Similarly, the two groups were comparable in terms of the SRF height at baseline and all the subsequent visits. However, the IRm arm had significantly lesser SFCT as compared to the BRm arm at baseline, which was maintained until week 52. Likewise, the proportion of eyes with IRF was significantly greater in the BRm eyes at baseline and week 12. However, the difference was no longer significant at weeks 24 and 52, respectively.
At week 52, the eyes treated with IRm showed a significant reduction in the SRF height as compared to the baseline. However, no significant difference was noted in the SFCT, and the proportion of eyes having IRF and SRF.
In the BRm arm, the SFCT reduced significantly at week 52. However, no significant difference was noted in the SRF height or the proportion of patients with IRF or SRF at week 52. Figures 1 and 2 illustrate the representative case examples of PCV cases treated with IRm and BRm, respectively. Tables 2 and 3 demonstrate the changes in the BCVA and SD-OCT parameters in both arms over 52 weeks. A comparison between the two groups at weeks 12, 24, and 52 is shown in Table 4.
Multimodal imaging of a 64-year-old female presenting with left eye reduction of vision (best-corrected visual acuity [BCVA]—20/200) demonstrated subretinal exudation in the parafoveal region with diffuse subfoveal pigment epithelial detachment (PED) on multicolor imaging (MCI) (a), a large conical PED with intraretinal fluid (IRF) and subretinal (SRF) on spectral-domain optical coherence tomography (SD-OCT) (c), and polypoidal lesions (PL) on the indocyanine green angiography (ICGA) (b). A diagnosis of polypoidal choroidal vasculopathy (PCV) was made and the patient underwent three loading doses of the innovator ranibizumab injection (IRm; LUCENTIS®). At 3 months, the BCVA improved to 20/80 with a significant reduction in IRF and SRF on the SD-OCT (d). The patient underwent further three IRm injections over the next 9 months. The IRF and SRF had completely resolved at months 6 (e) and 12 (f), respectively, with a reduction in the height of the PED and presence of subretinal hyperreflective material (SHRM) and a thin epiretinal membrane (ERM). Her final BCVA improved to 20/40 at 12 months
A 61-year-old male presented with a large subfoveal pigment epithelial detachment (PED) with exudation in the left eye (OS) on multicolor imaging (MCI; a) with the best-corrected visual acuity (BCVA) reducing to 20/120. An extra-large PED with subretinal fluid (SRF) was seen on the spectral-domain optical coherence tomography (SD-OCT; c) with a cluster of polypoidal lesions (PL) noted on the indocyanine green angiography (ICGA; b) at baseline. A diagnosis of polypoidal choroidal vasculopathy (PCV) was made and the patient underwent three loading doses of the biosimilar ranibizumab injection (BRm; Razumab®). At 3 months, complete resolution of the SRF was seen with a significant reduction in the PED height on the SD-OCT (d) and the BCVA improving to 20/40. After observation, the BCVA showed further improvement up to 20/32 at 6 months with a decrease in the PED height (e). The patient underwent one more dose of BRm over the next 6 months for recurrence of SRF. At 12 months, his BCVA improved to 20/20, with a dry macula and almost complete resolution of the PED (f)
Number of Injections
The mean number of injections in the IRm arm were significantly more than the BRm arm (IRm: 5.41 ± 0.94; BRm: 4 ± 1.45; P = 0.0004).
Safety Analysis
One eye in the BRm arm (5.26%) developed mild anterior uveitis which resolved with topical corticosteroid medications within 2 weeks. No other ocular or systemic adverse events were noted in either arm.
Discussion
PCV is an abnormality in choroidal vasculature which results in recurrent serous exudation and hemorrhages [14]. Furthermore, increased levels of VEGF are observed in the affected eyes of patients with PCV. Thus, anti-VEGF agents are a valuable treatment option for PCV. Anti-VEGF agents can reduce the IRF and the SRF and aid in the restoration of macular morphology and visual function. Although PDT has shown excellent results in PCV, its availability is quite challenging. Moreover, it is associated with a few disadvantages, such as the risk of choroidal ischemia and atrophy, RPE rips, hemorrhages (subretinal, vitreous, suprachoroidal), its unsuitability for the treatment of multiple, widely distributed lesions, and its poor results for patients with substantial PED or submacular hemorrhage.
The efficacy of the innovator ranibizumab molecule in PCV patients has been well established by the EVEREST and the LAPTOP [7, 8] studies. However, the role of the biosimilar agent Razumab in the management of PCV remains unexplored. Our study demonstrated the equivalence in efficacy between the BRm and IRm when administered for treatment of PCV, which has never been evaluated before. Both the two ranibizumab molecules showed no significant difference in visual acuity and residual fluid (IRF/SRF) at week 52 in our real-world study.
In our real-world study, the patients were treated on a PRN basis after the initial three loading doses of injection in both arms. Even though both medications displayed comparable efficacy in terms of vision after the loading dosage, the BRm eyes had more residual IRF than the IRm eyes. This could be attributable to a greater proportion of eyes with IRF at baseline in this group. The higher number of previously treated PCV eyes could explain this finding in the BRm arm at baseline. These eyes most likely had persistent IRF, and because the patented ranibizumab is expensive, patients may have chosen the more economical option, Razumab. This difference in residual IRF, on the other hand, was not found at weeks 24, and 52, respectively. This observation indicates that the BRm is effective at resolving the fluid, however, the response may be delayed. The presence of resistant PCV could also have influenced this observation as this group included significantly more eyes with previous anti-VEGF treatment in comparison to predominantly treatment naïve eyes in the IRm group. Further long-term prospective studies with this novel biosimilar (Razumab) in the management of treatment-naïve PCV will throw further insights into its efficacy on fluid resolution.
Neither of the ranibizumab molecules demonstrated any significant difference in visual acuity between them at all visits until week 52. Improvement in BCVA from the baseline until week 52 was noted in both the groups, although it did not attain statistical significance in the BRm eyes. The presence of a greater number of previously treated eyes and/or more eyes having IRF at baseline in the BRm group can account for the lack of significant visual improvement in these eyes. Indeed, the presence of IRF has a greater bearing on the final visual outcomes as demonstrated by multiple studies. Even though data on comparison of biosimilar ranibizumab with innovator ranibizumab in PCV do not exist; a comparison of both these agents in neovascular AMD has been reported. While one study involving the same biosimilar used in this study found similar efficacy with the reference ranibizumab at 8 weeks [15], the other comparative phase 3 study involving another biosimilar (SB11, Samsung Bioepis Co. Ltd, South Korea) demonstrated equivalence in safety and efficacy at 8 weeks in eyes with neovascular AMD [16]. At 12 months, the present comparative study has the longest follow-up evaluation.
PCV belongs to the pachychoroid disease spectrum characterized by increased CT and/or presence of underlying pachyvessels. An anti-VEGF agent with the ability to alter the choroidal morphometry can conceivably modify the underlying disease. In our study, the SFCT was reduced significantly at week 52 after treating with the biosimilar agent but not with the innovator molecule. These results are encouraging and further studies evaluating the changes in choroidal biomarkers on multimodal imaging after treating with the BRm are warranted to validate our findings.
While using anti-VEGF medicines, ocular and systemic safety remains a concern. Endophthalmitis caused by IRm and aflibercept has been estimated to be 0.35%. Simultaneously, the use of BRm has been fraught with difficulties due to the occurrence of sporadic episodes of sterile endophthalmitis. In our real-world study, the presence of mild intraocular inflammation (IOI) was seen in one eye (5.26%) after BRm injection which resolved with topical steroids. This is a concern with biosimilars where impurities such as endotoxin levels often influence these reactions. Though this problem is now less relevant due to the stringent quality processes, a distant concern remains. Monitoring these patients in the post-injection period is essential. No additional safety concerns, either ocular or systemic, were noted with the BRm- and IRm-treated patients.
The mean number of injections in the IRm arm was significantly greater than in the BRm eyes. Since the patients were advised PRN regimen after the initial loading doses, the likelihood of dropout is greater as compared to other regimens such as the treat-and-extend. Also, patients in the BRm arm had received a significantly greater number of injections prior to switching to the biosimilar agent. The chronicity of the condition in these BRm group eyes, as well as the related cumulative expense of treatment, meant that these patients were more likely to discontinue the therapy. In developing countries such as India, the expense of an anti-VEGF treatment regimen can be a major determinant of long-term compliance. The per-dose cost of the FDA-approved agents, including Lucentis ($320), Eylea ($760), and Beovu ($380), is quite high in India considering the low per-capita GDP ($1900). Although bevacizumab is more economical ($40 per dose), its compounding and labeling issues limit its broader acceptance. Approved biosimilars such as Razumab, which are cost-effective ($125) and packaged as a single-use vial, make them an attractive anti-VEGF agent in the retinal physician’s armamentarium. Biosimilar products can add to cost savings in health care systems and facilitate patients' access to therapy [17]. Therefore, a safe and effective biosimilar may decrease the cost of therapy and allow patients to have a greater chance of receiving a full-fledged standardized protocol of treatment with better compliance and therefore effective treatment regimen. Razumab seems to be well placed in this regard. In India, more than 200,000 Razumab injections had been administered as of December 2021. Since PCV is more prevalent in the Asian population, treatment options which are as effective and more economical than the previously practiced options are essential. We would like to recommend larger RCT’s to be conducted for further evaluating the efficacy and safety of biosimilar Razumab in PCV.
The generalizability of the outcomes from this study is upheld by its consistency with those of past studies of ranibizumab like EVEREST, LAPTOP, and PLANET study [7, 8, 11] in terms of mean changes in visual as well as anatomical outcomes. Although the randomized controlled trials provide a good platform for developing management regimens for vitreoretinal disorders, their broad use is limited because they may not fully reflect real-world delivery settings and population diversity. In PCV, proof from the real-world practice settings shows that patients might be undertreated and receive fewer anti-VEGF injections than recommended, irrespective of the treatment regimen. This results in lower efficacy than observed in clinical trial settings, as observed from our results.
The limitations of the current study include the retrospective design and limited sample size. Also, the details of the patients who had received previous treatments before the timeline was not captured. Furthermore, because the biosimilar arm has fewer treatment-naive patients than the innovator arm, the biosimilar arm may have had suboptimal outcomes. Despite these limitations, this study is the first to compare the safety and efficacy of the two licensed ranibizumab molecules in patients with PCV. The longer follow-up period of 1 year is a major strength of the study. Long-term prospective studies are proposed to further examine the function of BRm in the management of PCV, both treatment-naïve and resistant cases, in comparison to other anti-VEGF agents, namely IRm, Eylea, and Beovu.
Conclusions
In the management of PCV, our real-world data show that the ranibizumab biosimilar Razumab has equivalent visual acuity outcomes to the innovator ranibizumab molecule while maintaining an adequate safety profile. These encouraging results with this regulatory approved biosimilar drug may support its wider acceptance as an economical and efficacious alternative to the branded agents.
References
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10:1–8.
Sakurada Y, Yoneyama S, Imasawa M, Iijima H. Systemic risk factors associated with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Retina. 2013;33:841–5.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green video angiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–10.
Kwok AK, Lai TY, Chan CW, Neoh EL, Lam DS. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol. 2002;86:892–7.
Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121:1392–6.
Moorthy RS, Lyon AT, Rabb MF, Spaide RF, Yannuzzi LA, Jampol LM. Idiopathic polypoidal choroidal vasculopathy of the macula. Ophthalmology. 1998;105:1380–5.
Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64.
Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol. 2013;156:644–51.
Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010;94:297–301.
Lee SY, Kim JG, Joe SG, Chung H, Yoon YH. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2008;22(2):92–9.
Lee WK, Iida T, Ogura Y, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136:786–93.
Sharma S, Khan M, Chaturvedi A, RE-ENACT 2 Study Investigators Group. A multicenter, retrospective study (RE-ENACT 2) on the use of Razumab (World’s First Biosimilar Ranibizumab) in wet age-related macular degeneration. Ophthalmol Ther. 2020;9(1):103–14.
Sharma S, Khan M, Chaturvedi A, RE ENACT Study Investigators Group. Real-life clinical effectiveness of Razumab (world’s first biosimilar ranibizumab) in wet age-related macular degeneration, diabetic macular edema, and retinal vein occlusion: a retrospective pooled analysis. Int J Ophthalmol Eye Res. 2018;6(4):377–83.
Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29:19–29.
Sharma A, Kumar N, Parachuri N. Ranibizumab biosimilar (Razumab) vs innovator Ranibizumab (Lucentis) in neovascular age-related macular degeneration (n-AMD)—efficacy and safety (BIRA study). Eye. 2021. https://doi.org/10.1038/s41433-021-01616-9.
Woo SJ, Veith M, Hamouz J, et al. Efficacy and safety of a proposed ranibizumab biosimilar product vs a reference ranibizumab product for patients with neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2021;139:68–76.
Sharma A, Kumar N, Bandello F, Loewenstein A, Kuppermann BD. Need of education on biosimilars amongst ophthalmologists: combating the nocebo effect. Eye. 2020;34:1006–7.
Acknowledgements
We thank all study participants for their involvement in the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Jay Sheth, Indu Nair, and Manoj Soman conceived and designed the work. Jay Sheth performed the data analysis, interpretation of the results, and drafting of the manuscript. Manoj Soman and Unnikrishnan Nair performed critical revision of the manuscript. All authors read and approved the final manuscript.
Disclosures
Manoj Soman, Indu Nair, Jay Sheth, and Unnikrishnan Nair declare that they have no conflict of interest related to this work.
Compliance with Ethics Guidelines
Written informed consent for treatment and data collection was obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of Chaithanya Eye Hospital in Trivandrum, India.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Soman, M., Nair, I., Sheth, J.U. et al. Innovator Versus Biosimilar Ranibizumab in Polypoidal Choroidal Vasculopathy: Real-World Evidence. Ophthalmol Ther 11, 1175–1186 (2022). https://doi.org/10.1007/s40123-022-00507-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00507-w