Abstract
Introduction
The REal life assessmENt of safety And effeCTiveness of Razumab (RE-ENACT) and long-term RE-ENACT 2 retrospective studies have evaluated the use of Razumab™ (world’s first biosimilar ranibizumab) in retinal disorders in Indian patients. This report presents the subgroup analysis from the RE-ENACT 2 study in patients with wet age-related macular degeneration (wet AMD).
Methods
Medical charts of patients administered biosimilar ranibizumab injections as PRN treatment regimen between September 2015 and June 2018, at 17 centers across India, were reviewed. Changes from baseline in best-corrected visual acuity (BCVA, based on Snellen’s or logMAR chart), central subfield thickness (CSFT), intraocular pressure (IOP), and proportions of patients having intraretinal fluid (IRF) and subretinal fluid (SRF) at weeks 4, 8, 12, 16, 20, 24, 30, 36, and 48 were evaluated.
Results
Of 103 patients with wet AMD, 62.1% were men and the majority (74.8%) were treatment naïve. The majority (57.9%) of the patients had received 3 (range 1–5) injections. Significant improvements were observed from baseline to all timepoints for BCVA (baseline, 0.92 ± 0.6 [n = 94]; week 48, 0.51 ± 0.4 [n = 14]; P = 0.0014) and CSFT (baseline, 430.83 ± 14.4 [n = 85]; week 48, 301.26 ± 11.6 [n = 15]; P < 0.0001). Changes in IOP from baseline to 48 weeks were minimal and not significant (14.92 ± 3.2 [n = 94] vs. 14.50 ± 2.1 [n = 18]; P = 0.9068). A decrease in proportions of patients having IRF (baseline, 63.6% [n = 99] vs. week 48, 15% [n = 20]) and SRF (baseline, 82.3% [n = 96] vs. week 48, 5% [n = 20]) were also observed. Similar results were observed for occult and classic subgroups. There were no new safety concerns.
Conclusion
Razumab (biosimilar ranibizumab) demonstrated improvements in visual acuity and disease outcomes in patients with wet age-related macular degeneration without new safety issues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
AMD accounts for 1.7–3.3% of all blindness diagnosed in India. |
Ranibizumab is an agent approved by the US Food and Drug Administration (FDA) and European Medicine Agency (EMEA) for the treatment of wet AMD. |
Biosimilar ranibizumab, Razumab™, approved by the Drug Controller General of India (DCGI) in 2015, provides a cost-effective alternative, which is accessible to Indian patients. |
This report presents the effectiveness of Razumab in patients with wet AMD treated in a real-world setting. |
Razumab (biosimilar ranibizumab) demonstrated improvements in visual acuity and disease outcomes in patients with wet AMD without new safety issues. |
Introduction
The third most common cause of blindness (after cataract and glaucoma), age-related macular degeneration (AMD, 8.7% of all blindness), affects the macular region of elderly patients (over 60 years old) [1, 2]. Wet AMD is characterized by neovascularization spreading to the subretinal pigment epithelium or subretinal space, and increased presence of intraretinal fluid (IRF) or subretinal fluid (SRF) [3,4,5,6]. AMD is classified as “wet” and “dry” types [2]. Dry AMD precedes wet AMD (also known as neovascular AMD) through choroidal neovascularization (CNV), in which abnormal blood vessels from the choroid develop in or under the retina [7]. CNV and related exudation, bleeding, and disciform scarring are responsible for more than 80% of vision loss in AMD. Wet AMD can be further categorized into classic, occult, or mixed types [8].
Of all patients with AMD, 10–15% cases are wet AMD, which accounts for approximately 90% of blindness due to this disease [9]. In developing countries like India, a rapid increase in ageing population may be an indicator of emerging public health problems associated with old age such as AMD (prevalence 3.1–10.6%) [10]. Population-based surveys have revealed that AMD accounts for 1.7–3.3% of all blindness diagnosed in India [11].
Vascular endothelial growth factor (VEGF) is an important cytokine in the pathogenesis of CNV. Anti-VEGF agents have shown favorable treatment outcomes, as compared with the previous treatment options like thermal laser photocoagulation and photodynamic therapy (PDT) with intravenous verteporfin, and have become the standard treatment for wet AMD [1, 12,13,14].
Ranibizumab is approved by the US Food and Drug Administration (FDA) and European Medicine Agency (EMEA) for the treatment of wet AMD. The efficacy and safety of ranibizumab in the treatment of several retinal disorders including wet AMD have been established in earlier prospective, randomized, double-masked clinical trials. The US FDA approval of ranibizumab for the treatment of visual impairment due to wet AMD was based on the results from the two pivotal phase 3 MARINA [15] and ANCHOR [16] studies.
The world’s first biosimilar ranibizumab Razumab™ was developed by Intas Pharmaceuticals Ltd. to provide a cost-effective alternative which is accessible to patients with retinal disorders [17], and it was approved by the highest Indian regulatory body, the Drug Controller General of India, in 2015. A prospective study has demonstrated the efficacy and safety of biosimilar ranibizumab in Indian patients with chorioretinal vascular diseases including wet AMD [18].
A previous retrospective, multicenter, observational study, REal life assessmENt of safety And effeCTiveness of Razumab (RE-ENACT), established the use of biosimilar ranibizumab for the treatment of retinal disorders in a real-world clinical setting [19,20,21]. The RE-ENACT 2 study generated long-term data on the use of biosimilar ranibizumab. The current report presents the subgroup analysis of the RE-ENACT 2 study in Indian patients with wet AMD.
Methods
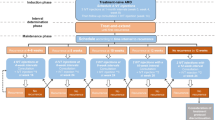
Data of patients with wet AMD of either sex, aged at least 18 years old, who received biosimilar ranibizumab injections (0.5 mg or 0.05 mL as PRN regimen) for the treatment of wet AMD between September 2015 and June 2018 at 17 centers across India were retrospectively analyzed. Wet AMD was defined and clinically diagnosed by the presence of choroidal neovascular membrane along with its sequelae, such as focal areas of retinal pigment epithelium (RPE) loss, subretinal or sub-RPE hemorrhage or serous fluid, and subretinal fibrosis. Both, treatment-naïve and patients previously treated with other anti-VEGF/steroids/laser treatment were included in this study. Patients in whom assessment of optical coherence tomography (OCT) results were not available (i.e., dense cataract) were excluded. Razumab (biosimilar ranibizumab) PRN treatment regimen was guided by disease activity criteria. Disease activity was defined as visual impairment attributable to increase in intraretinal or subretinal fluid, or active leakage from the CNV lesion as compared to the previous visit findings. Razumab treatment was discontinued if no disease activity was observed. The treatment was resumed if the disease activity criteria were fulfilled.
Ethics committee approval was obtained to conduct this retrospective study (OM Ethics Committee, Ahmedabad, Gujarat, India) and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
The study endpoints included change from baseline to weeks 4, 8, 12, 16, 20, 24, 30, 36, and 48 in the best corrected visual acuity (BCVA, measured by Snellen’s chart or the logMAR chart), central subfield thickness (CSFT, measured by spectral-domain OCT [SD-OCT]), and intraocular pressure (IOP). The decrease in the proportion of patients with IRF and SRF, measured by SD-OCT, from baseline to each timepoint was also evaluated. Two-tailed paired t test was used to analyze BCVA and CSFT, while χ2 test was used for IRF and SRF. All statistical analyses were done using SAS® 9.4.
Results
Patient Disposition and Demographics
A total of 103 patients with wet AMD were included in this subgroup analysis; 62.1% were men and the majority (74.8%) were treatment naïve. The majority of the patients had received 3 (range 1–5) biosimilar ranibizumab injections (73.8%) and the majority of the patients had hypertension (51.5%) and diabetes (22.3%). The most common wet AMD subtype was classic (n = 72, mean age 67.4 ± 8.4 years, men 62.5%) followed by occult (n = 13, mean age 63.6 ± 12.2 years, men 46.2%) and minimally classic (n = 8, mean age 65 ± 9.7 years, men 75%). The baseline BCVA values were available in 94 (classic 66, occult 13, minimally classic 4) patients and CSFT in 85 (classic 69, occult 13, minimally classic 3) patients, respectively. The treatment effects were observed up to 48 weeks. Table 1 represents the patient disposition and baseline characteristics.
Best Corrected Visual Acuity
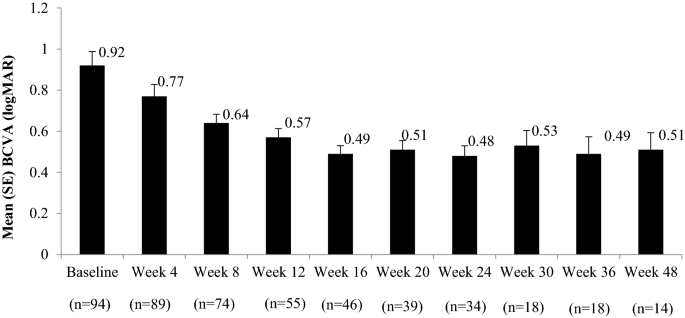
The mean ± SE BCVA improved significantly (P < 0.05) from baseline to all timepoints (baseline, 0.92 ± 0.06; week 48, 0.51 ± 0.08) indicating improved visual acuity. A slight decrease in BCVA improvements were observed from weeks 20 to 30 compared to previous weeks, though these improvements were significant as compared with baseline; thereafter improvements were again observed till the last visit (Fig. 1). Figure 2 represents the mean change in BCVA from baseline at each timepoint. The majority (73.8%) of the patients had received three biosimilar ranibizumab injections with significant improvements (P < 0.05) seen in BCVA at all timepoints. A maximum of five injections were administered and the improvements were observed up to 48 weeks. The change in mean BCVA from baseline to all timepoints did not differ significantly when evaluated for treatment-naïve vs. previously treated patients (Online Resource 1), except at weeks 4 and 20, where the changes were significant in favor of treatment-naïve patients.
The improvements in the BCVA were significant (P < 0.05) from baseline to 48 weeks for classic (baseline, 0.98 ± 0.08; week 48, 0.50 ± 0.1) and occult (baseline, 0.69 ± 0.18; week 48, 0.54 ± 0.24) subgroups (Fig. 3).
Central Subfield Thickness
A significant (P < 0.05) decrease in the CSFT scores indicating improved disease condition was observed from baseline (430.83 ± 14.4) to 48 weeks (301.26 ± 11.6) (Fig. 4). Figure 5 represents the mean change in CSFT from baseline at each timepoint. Improvements in CSFT were continuous till the 20th week (5th injection), after which a slight decrease in the improvement was observed compared to previous weeks. There were significant improvements (P < 0.05) in CSFT from baseline to all timepoints in patients who received three biosimilar ranibizumab injections. The change in mean CSFT from baseline to all timepoints did not differ significantly when evaluated for treatment-naïve vs. previously treated patients (Online Resource 2), except at weeks 8 to 20, where the changes were significant in favor of treatment-naïve patients.
The decrease in CSFT was significant (P < 0.05) from baseline to 48 weeks when analyzed for classic (449.67 ± 18.26 vs. 306.40 ± 12.46) and occult subgroups (375.08 ± 20.63 vs. 275.00 ± 42.45) (Fig. 6).
Intraretinal Fluid and Subretinal Fluid
A significant (P < 0.05) reduction in the proportion of patients having IRF or SRF from baseline to all timepoints was observed, indicating improved disease condition. The proportions of patients having IRF at baseline and at weeks 4, 8, 12, 16, 20, 24, 30, 36, and 48 were 63.6%, 46.3%, 39.5%, 33.8%, 19%, 12.5%, 15%, 3.8%, 9.5%, and 15%, respectively. Similarly, the proportions of patients having SRF at baseline and at weeks 4, 8, 12, 16, 20, 24, 30, 36 and 48 were 82.3%, 69.2%, 44.7%, 28.8%, 6.9%, 10.4%, 12.5%, 11.5%, 4.8%, and 5%, respectively. The proportions of patients with IRF and SRF present or absent at each follow-up are presented in Online Resource 3.
Intraocular Pressure
The changes in mean IOP scores observed from baseline (14.92 ± 3.2 [n = 94]) to 48 weeks (14.50 ± 2.1 [n = 18]) were ± 1 mmHg at most of the timepoints, which were not significant. Similarly, the changes were not significant from baseline to all timepoints in patients who received three biosimilar ranibizumab injections. When evaluated for treatment-naïve vs. previously treated patients, changes in mean IOP from baseline to all timepoints did not differ significantly. The changes in IOP from baseline were minimal, and not significant, when analyzed for classic and occult subgroups.
Discussion
Wet AMD is a chronic, progressive disease of the central retina and a major cause of irreversible vision loss worldwide [22]. In wet AMD, overexpression of VEGF is known to be associated with neoangiogenesis, which is related to the pathogenesis of wet AMD [22, 23]. Ranibizumab, a recombinant humanized monoclonal antibody fragment (Fab), can inhibit the growth of blood vessels by binding to all isoforms of VEGFA [23]. Improvements in visual acuity have been reported with ranibizumab to a greater extent than standard treatments such as laser and photodynamic therapy with verteporfin [24].
Ranibizumab was associated with significant improvements in visual acuity and was well tolerated in the treatment of wet AMD in two pivotal phase 3 MARINA [15] and ANCHOR studies [16]. The regulatory approval of ranibizumab for wet AMD was based on these studies, which showed improvement in visual acuity over 12 months with monthly dosing.
Razumab, the world’s first biosimilar ranibizumab, has shown effectiveness for various vitreoretinal disorders in both prospective [18] and retrospective real-world studies [19]. The RE-ENACT (n = 561) study, a multicenter, retrospective study, strengthened the effectiveness of biosimilar ranibizumab in vitreoretinal disorders in Indian patients in routine clinical settings. Significant improvements were seen with various visual parameters including BCVA, central macular thickness, IRF, and SRF with biosimilar ranibizumab injections (three injections) for 3 months.
The RE-ENACT study had a short-term (12 weeks) treatment duration and evaluated the use (effectiveness measured by improvements in BCVA, CMT, IRF, and SRF) of biosimilar ranibizumab in wet AMD, diabetic macular edema (DME), and retinal vein occlusion (RVO) in routine clinical settings. The RE-ENACT 2 (n = 341) study was conducted to evaluate the use (effectiveness measured by improvements in BCVA, CSFT, IRF, and SRF) of biosimilar ranibizumab for a longer term (48 weeks) in patients with wet AMD, DME, RVO and patients with myopic choroidal neovascularization (CNV), who were not evaluated in the previous RE-ENACT study. Additionally, the RE-ENACT 2 study evaluated the patients who had received up to five biosimilar ranibizumab injections, whereas the previous RE-ENACT study evaluated the patients who had received three biosimilar ranibizumab injections. The current report presents the subgroup analysis of the RE-ENACT 2 study in patients with wet AMD, which demonstrated significant improvements in visual acuity and disease outcomes with biosimilar ranibizumab.
In this study, significant improvements in the logMAR BCVA scores after biosimilar ranibizumab treatment from baseline to 48 weeks were observed, consistent with the subgroup analysis of the RE-ENACT study in patients with wet AMD, which showed significant improvements in BCVA from baseline to 12 weeks [21]. The subgroup analysis in the current report demonstrated significant improvements in BCVA values in both classic and occult patients. The long-term sustained benefits of ranibizumab in the treatment of wet AMD were reported in the MARINA and ANCHOR studies. Improvements in visual acuity with ranibizumab treatment was reported at 12 months to 24 months in the randomized, double-blind MARINA trial (n = 716) [15], and at 12 months in the randomized, double-blind ANCHOR trial (n = 423) [16]. A single-center, retrospective, observational study (n = 121) showed significant improvements (P < 0.001) in mean BCVA from baseline to 12 months (43.2 ± 19.3 vs. 51.7 ± 20.1) in patients with wet AMD [25]. Significant improvement in logMar BCVA with ranibizumab treatment was reported by Figurska and Stankiewicz from baseline (0.73 ± 0.27) to 12 months (0.58 ± 0.26) in 25 patients with exudative AMD, where only three injections of ranibizumab were administered [26]. Another retrospective study showed a significant increase in the mean BCVA scores from baseline to 12 months (0.78 ± 0.33 vs. 0.61 ± 0.39, P < 0.001) in patients with exudative AMD (n = 79) [27]. These results are similar to those observed in our study where the majority of the patients received three biosimilar ranibizumab injections and the improvements in BCVA were observed up to 48 weeks.
Ranibizumab treatment is associated with improvement of vision accompanied by a stabilization or reduction in CSFT [28]. The subgroup analysis in both classic and occult patients demonstrated similar results in this study with a significant reduction in the CSFT values. Significant reductions in CSFT with biosimilar ranibizumab were observed in this study at all timepoints starting at 4 weeks to 48 weeks. The mean change from baseline to 48 weeks in CSFT was − 129.57 µm, whereas the TREND study showed a mean change of − 173.3 μm in CSFT with monthly ranibizumab treatment (n = 287). The improvements in CSFT were reported throughout the 12-month follow-up period [29], similar to that observed in our study. The phase 3, double-masked MINERVA study showed a comparable mean change of − 102.7 µm in CSFT at 12 months with ranibizumab treatment (n = 119) [28].
An increase in IOP is reported after ranibizumab injection in patients with wet AMD [30]. Freund and colleagues reported less than 1 mmHg change in the IOP from baseline to 48 weeks, which lasted up to 96 weeks of follow-up, in the VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD (VIEW) 1 and 2 studies, which were randomized, active-controlled phase 3 trials (n = 2457) [31]. Similar results with minimal change in IOP (± 1 mmHg at most timepoints) were observed in our study.
According to the Comparison of Age-related Macular Degeneration Treatments Trials Research Group, ranibizumab treatment effectively reduced the proportion of patients with IRF and SRF starting at 4 weeks, which was maintained up to 2 years of follow-up [32]. A multicenter, randomized, 24-month, phase 4, single-masked, non-inferiority clinical trial by FLUID investigators demonstrated a decline in the IRF and SRF with ranibizumab treatment throughout the study period of 2, 12, and 24 months [33], similar to the results obtained in the current report with decrease in the proportion of patients with IRF and SRF throughout the study.
Overall, there were no significant differences in BCVA, CSFT, IOP, IRF, and SRF when evaluated for treatment-naïve vs. previously treated patients. Overall, this subgroup analysis demonstrated no new safety concerns with biosimilar ranibizumab in patients with wet AMD than those reported with innovator ranibizumab.
The major limitation of this study included its retrospective nature because of which complete information pertaining to previous treatments, ischemia, bilaterality and severity of the disease, and adverse events could not be presented as they were not captured in the medical records. Also, the data on effectiveness parameters were not available for all patients at all timepoints, and the number of patients at each timepoint is mentioned in the respective figures. Overall, no new safety concerns compared to the innovator ranibizumab were observed. The visual acuity was measured with logMAR/Snellen’s charts in this study, which is considered inferior to the Early Treatment Diabetic Retinopathy Study (ETDRS) charts, used commonly in controlled clinical studies [34].
Conclusions
The current subgroup analysis of patients with wet AMD from the RE-ENACT 2 study showed improvements in all parameters including best corrected visual acuity, central subfield thickness, and intraretinal and subretinal fluids. Even with intravitreal injections of biosimilar ranibizumab, there was no clinically significant increase in intraocular pressure. Though the majority of the patients received only three injections of biosimilar ranibizumab, the improvements lasted up to almost a year, which reinforces Razumab, the world’s first biosimilar of ranibizumab, as an effective treatment option in managing wet age-related macular degeneration.
Change history
22 December 2021
The license text was incorrectly structured. The article has been corrected.
References
Alexandru MR, Alexandra NM. Wet age related macular degeneration management and follow-up. Rom J Ophthalmol. 2016;1:9–13.
Ayoub T, Patel N. Age-related macular degeneration. J R Soc Med. 2009;2:56–61.
Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;4:564–72.
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16.
Jaffe GJ, Martin DF, Toth CA, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials (CATT). Ophthalmology. 2013;9:1860–70.
Topal T, Kar T, Yıldırım Y, et al. Evaluation of aflibercept treatment responses in eyes with bevacizumab/ranibizumab-resistant wet age-related macular degeneration. Turk J Ophthalmol. 2017;3:133–7.
Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;1:26–39.
Cohen SY, Creuzot-Garcher C, Darmon J, et al. Types of choroidal neovascularisation in newly diagnosed exudative age-related macular degeneration. Br J Ophthalmol. 2007;9:1173–6.
Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;11:1640–2.
Thapa R, Bajimaya S, Paudyal G, et al. Prevalence of and risk factors for age-related macular degeneration in Nepal: the Bhaktapur Retina Study. Clin Ophthalmol. 2017;11:963–72.
Woo JH, Sanjay S, Au Eong KG. The epidemiology of age-related macular degeneration in the Indian subcontinent. Acta Ophthalmol. 2009;3:262–9.
Sivaprasad S, Hykin P. What is new in the management of wet age-related macular degeneration? Br Med Bull. 2013;1:201–11.
Regillo CD. Anti-VEGF maintenance therapy for neovascular AMD. Retina Today. 2014;63–67.
Jaki Mekjavic P, Zaletel Benda P. Outcome of 5-year treatment of neovascular age-related macular degeneration with intravitreal anti-VEGF using “treat and extend” regimen. Front Med (Lausanne). 2018;5:125.
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;14:1419–31.
Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;14:1432–44.
Intas Pharmaceuticals Limited. Intas launches RAZUMAB, globally the first biosimilar to Lucentis® (ranibizumab). https://www.prnewswire.com/in/news-releases/intas-launches-razumab-globally-the-first-biosimilar-to-lucentis-ranibizumab-508383021.html. Accessed 12 Jul 2019.
Sameera VV, Ayachit A, Joshi S, Guruprasad AS. Safety and efficacy of Razumab—the new biosimilar in India: our experience. Kerala J Ophthalmol. 2016;28:180.
Sharma S, Khan MA, Chaturvedi A, RE-ENACT Study Investigators Group. Real-life clinical effectiveness of Razumab® (the world’s first biosimilar of ranibizumab) in retinal vein occlusion: a subgroup analysis of the pooled retrospective RE-ENACT study. Ophthalmologica. 2019;241:24–31.
Sharma S, RE-ENACT Study Investigators Group, Khan MA, Chaturvedi A. Real-life clinical effectiveness of Razumab® (world’s first biosimilar ranibizumab) in wet age-related macular degeneration, diabetic macular edema, and retinal vein occlusion: a retrospective pooled analysis. Int J Ophthalmol Eye Res. 2018;6(4):377–83.
Sharma S, RE-ENACT Study Investigators Group, Khan MA, Chaturvedi A. Real life clinical effectiveness of Razumab® (world’s first biosimilar ranibizumab) in wet age-related macular degeneration: a subgroup analysis of pooled retrospective RE-ENACT study. Int J Ophthalmol Eye Res. 2018;6(2):368–73.
Chong V. Ranibizumab for the treatment of wet AMD: a summary of real-world studies. Eye (Lond). 2016;2:270–86.
Ferro Desideri L, Barra F, Ferrero S, Traverso CE, Nicolo M. Clinical efficacy and safety of ranibizumab in the treatment of wet age-related macular degeneration. Expert Opin Biol Ther. 2019;1–17.
Blick SK, Keating GM, Wagstaff AJ. Ranibizumab. Drugs. 2007;8:1199–206 (discussion 1207–9).
Qi H-J, Li X-X, Zhang J-Y, Zhao M-W. Efficacy and safety of ranibizumab for wet age-related macular degeneration in Chinese patients. Int J Ophthalmol. 2017;1:91–7.
Małgorzata F, Stankiewicz A. Effectiveness of ranibizumab intravitreal injections for exudative age-related macular degeneration treatment: 12-month outcomes. Med Sci Monit. 2011;9:CR485–90.
Querques G, Azrya S, Martinelli D, et al. Ranibizumab for exudative age-related macular degeneration: 24-month outcomes from a single-centre institutional setting. Br J Ophthalmol. 2010;3:292–6.
Lai TYY, Staurenghi G, Lanzetta P, et al. Efficacy and safety of ranibizumab for the treatment of choroidal neovascularization due to uncommon cause: twelve-month results of the MINERVA study. Retina. 2018;8:1464–77.
Silva R, Berta A, Larsen M, Macfadden W, Feller C, Mones J. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;1:57–65.
Sharei V, Hohn F, Kohler T, Hattenbach LO, Mirshahi A. Course of intraocular pressure after intravitreal injection of 0.05 mL ranibizumab (Lucentis). Eur J Ophthalmol. 2010;1:174–9.
Freund KB, Hoang QV, Saroj N, Thompson D. Intraocular pressure in patients with neovascular age-related macular degeneration receiving intravitreal aflibercept or ranibizumab. Ophthalmology. 2015;9:1802–10.
Sharma S, Toth CA, Daniel E, et al. Macular morphology and visual acuity in the second year of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;4:865–75.
Guymer RH, Markey CM, McAllister IL, Gillies MC, Hunyor AP, Arnold JJ. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;5:723–34.
Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (an AOS thesis). Trans Am Ophthalmol Soc. 2009:311–324.
Acknowledgments
The authors thank the participants of this retrospective study.
RE-ENACT 2 Study Investigators Group: Dr. Manjunath Bhaskar Anandkumar, MS, Ganesh Netralaya, Sirsi, Karnataka, India, 581401, Email: manj009@gmail.com. Dr. Sangita Jain, MS, Dev Bhumi Superspeciality Hospital, Dehradun, Uttarakhand, India, 248001 Email: sangitavrs@yahoo.co.uk. Dr. Hemanth Murthy, MD, Retina Institute of Karnataka, Bangalore, Karnataka, India, 560018, Email: hemanthmurthy@yahoo.com. Dr. Naveenam Srinivasa Murlidhar, MD, Retina Institute of Karnataka, Bangalore, Karnataka, India, 560018, Email: retina.nsm@gmail.com. Dr. Raj Shri Hirawat, MD, Gomabai Netralaya, Neemuch, Madhya Pradesh, India, 458441, Email: drrajshreehirawat@gmail.com. Dr. Aditya Sudhalkar, MS, Sudhalkar Eye Hospital, Baroda, Gujarat, India, 390001, Email: adityasudhalkar@yahoo.com. Dr. Amarendra Deka, MS, Mission Netralaya, Shillong, Meghalaya, India, 793014, Email: dradeka@icloud.com. Dr. Alay Banker, MS, Banker Retina Clinic & Laser Centre, Ahmedabad, Gujarat, India, 380009, Email: alay.banker@gmail.com. Dr. Vatsal Parikh, MS, Drushti Eye And Retina Centre, Mumbai, Maharashtra, India, 400004, Email: vatsal@drushti.com. Dr. Manisha Agarwal, MS, Dr. Shroff Charity Eye Hospital, New Delhi, India, 110002, Email: agarwalmannii@yahoo.co.in. Dr. Charu Mithal, MS, Visitech Jasola Eye Centre, New Delhi, India, 110025, Email: drcharu_mithal@yahoo.co.in. Dr. Rajender Pal Singh, MD, Visitech Jasola Eye Centre, New Delhi, India, 110025, Email: jasola.visitech@gmail.com. Dr. Deepti Kulkarni, DNB, Ameya Laser & Research Pvt. Ltd., Miraj, Maharashtra, India, 416410, Email: deepti.ameya@gmail.com. Dr. Abhishek Desai, FRCS, Shri Ganpati Netralaya, Jalna, Maharashtra, India, 431203, Email: drabhisid@gmail.com. Dr. Rushikesh Naigaonkar, DNB, Shri Ganpati Netralaya, Jalna, Maharashtra, India, 431203, Email: rushikesh.naigaonkar@netralaya.org. Dr. Nishikant Borse, MS, Insight Eye Clinic, Mumbai, Maharashtra, India, 400014, Email: nishikantborse@yahoo.com. Dr. Simanta Pradeep Saikia, MS, Mission Netralaya, Shillong, Meghalaya, India, 793014, Email: jeetsaikia@yahoo.com. Dr. Atul kumar Sahu, MS, RKN Eye Care, Varanasi, Uttar Pradesh, 221010, India, Email: atulkrsahu@gmail.com. Dr. Shobhna Mange, MS; Shivam Retina Clinic, Surat, Gujarat, India, 395001, Email: shobha_72@yahoo.com. Dr. Arup Chakraborty, MS, Amulya Jyoti Eye Foundation, Kolkata, West Bengal, India, 700029, Email: drarupchak@gmail.com. Dr. Suprakash Roy, MS, Nivedita Eye Care And Microsurgery Centre, Bankura, West Bengal, India, 722141, Email: dr.suprakashroy@gmail.com. Dr. Valensha Surong, FRCS, Mission Netralaya, Shillong, Meghalaya, India, 793014, Email: valenshasurong@yahoo.com.
Funding
RE-ENACT 2 Study Investigator Group received an honorarium from Intas Pharmaceuticals Ltd. for their patient data contribution. Intas Pharmaceuticals Ltd supported the journal’s rapid service fee. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
The authors thank Mr. Shreekant Sharma (ISMPP CMPP™, Lambda Therapeutic Research Ltd.) for providing writing assistance and Dr. Venugopal Madhusudhana (ISMPP CMPP™, Lambda Therapeutic Research Ltd.) for additional editorial assistance for the development of this manuscript. The authors also thank Ms. Sudipta Ghosh (Biostatistics & Programming, Lambda Therapeutic Research Ltd.) for statistical analysis. The medical writing and statistical analysis services were funded by Intas Pharmaceuticals Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Dr. Shashikant Sharma is an employee of Intas Pharmaceuticals Ltd, Ahmedabad, India. Mujtaba Khan is an employee of Intas Pharmaceuticals Ltd, Ahmedabad, India. Alok Chaturvedi is an employee of Intas Pharmaceuticals Ltd, Ahmedabad, India.
Compliance with Ethics Guidelines
Ethics committee approval was obtained to conduct this retrospective study (OM Ethics Committee, Ahmedabad, Gujarat, India) and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Members of the RE-ENACT 2 Study Investigators Group are listed in the Acknowledgments.
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11316770.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sharma, S., Khan, M., Chaturvedi, A. et al. A Multicenter, Retrospective Study (RE-ENACT 2) on the Use of Razumab™ (World’s First Biosimilar Ranibizumab) in Wet Age-Related Macular Degeneration. Ophthalmol Ther 9, 103–114 (2020). https://doi.org/10.1007/s40123-019-00228-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-019-00228-7