Abstract
Introduction
This analysis of two Japanese clinical trials evaluated efficacy and safety after galcanezumab (GMB) discontinuation in patients with episodic migraine (EM) and chronic migraine (CM).
Methods
Data were from a 6-month, randomized, double-blind, placebo [PBO]-controlled primary trial (patients with EM) and a 12-month open-label extension trial (patients with EM/CM). Patients received 6 months’ (primary) or 12/18 months’ (extension) treatment with GMB 120 mg (GMB120) plus 240-mg loading dose or 240 mg (GMB240) with 4 months’ post-treatment follow-up. Efficacy was assessed as number of monthly migraine headache days during post-treatment. Safety was assessed via post-treatment-emergent adverse events (PTEAEs).
Results
The analysis population included 186 patients from the primary trial (PBO N = 93; GMB120 N = 45; GMB240 N = 48), 220 patients with EM from the extension trial (PBO/GMB120 N = 57; PBO/GMB240 N = 55; GMB120/GMB120 N = 55; GMB240/GMB240 N = 53), and 55 patients with CM (GMB120 N = 28; GMB240 N = 27). In patients with EM receiving 6 months’ GMB120, mean standard deviation (SD) monthly migraine headache days increased from 5.69 (4.64) at treatment end to 6.24 (4.37) at end of follow-up but did not return to pre-treatment levels (8.80 [2.96]). In the extension trial, mean monthly migraine headache days in patients with EM receiving GMB120 were 4.13 (3.85) after 12 months and 4.45 (3.78) at end of follow-up, and 3.59 (3.48) after 18 months and 3.91 (3.57) at end of follow-up. Monthly migraine headache days in patients with CM (12 months’ GMB120) were 10.71 (4.61) at treatment end and 11.17 (5.64) at end of follow-up (pre-treatment 20.15 [4.65]). Similar results were seen for patients receiving GMB240. The most observed PTEAE after GMB discontinuation was nasopharyngitis.
Conclusion
Galcanezumab exhibited post-treatment efficacy for up to 4 months in Japanese patients with EM and with CM. No unexpected safety signals were observed.
Clinical Trial Registration
ClinicalTrials.gov, NCT02959177 and NCT02959190.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Galcanezumab (GMB), a humanized monoclonal antibody that binds to the neuropeptide calcitonin gene-related peptide, is a preventive treatment for migraine with demonstrated efficacy and safety. |
In global trials in people with episodic migraine (EM), GMB showed reduced effect after discontinuation, but monthly migraine headache days did not return to baseline levels; this effect has not previously been examined in Japanese people with migraine. |
This analysis of data from two Japanese clinical trials evaluated efficacy and safety after GMB discontinuation in patients with EM and in patients with chronic migraine (CM) and identified clinical factors associated with increases in monthly migraine headache days after GMB discontinuation. |
What was learned from this study? |
Monthly migraine headache days did not return to pre-treatment levels for up to 4 months post-treatment in patients with EM and in patients with CM after GMB discontinuation. |
In patients with EM, an increase in monthly migraine headache days during the post-treatment phase after 6 months of treatment was associated with a lower number of headache days per month at baseline and longer disease duration; after 12 or 18 months of treatment, increased monthly migraine headache days during the post-treatment phase were associated with a higher aura frequency at baseline and a lower number of migraine headache days at treatment end. |
The demonstrated post-treatment effect of GMB is important for patients with migraine who may need to temporarily cease preventive medication. |
Introduction
Migraine is a debilitating chronic neurological condition, characterized by pulsating or pounding headaches and associated symptoms including nausea, photophobia, and phonophobia [1]. Pharmacological treatment of migraine includes both acute management during attacks and preventive therapy. However, traditional migraine preventive treatments have high discontinuation rates due to intolerance and lack of efficacy [2,3,4]. Some newer preventive treatments target the neuropeptide calcitonin gene-related peptide (CGRP) or its receptor. CGRP is implicated in the underlying pathophysiology of migraine and has been demonstrated to contribute to neurogenic inflammation, vasodilation, and transmission of painful stimuli [5]. Galcanezumab is a humanized monoclonal antibody that inhibits CGRP-mediated effects by binding to CGRP, thus preventing it from binding to its receptor [6]. The efficacy and safety of galcanezumab have been demonstrated in phase 2 and 3 randomized controlled trials for the prevention of migraine, including treatment-resistant migraine [7,8,9,10,11].
Anti-CGRP antibody treatments for migraine have demonstrated sustained response even after discontinuation in some people with migraine [12,13,14,15]. Results from two global randomized phase 3 trials of galcanezumab showed reduced effect in post-treatment phases, but monthly migraine headache days did not return to baseline levels [12]. In Japanese people with migraine, the efficacy and safety of galcanezumab have been demonstrated for episodic migraine (EM) in a phase 2 randomized controlled trial [9], and for EM and chronic migraine (CM) in a long-term, 12-month, open-label extension study [16]. However, continued efficacy of galcanezumab after discontinuation in Japanese patients has not yet been demonstrated. Furthermore, there is a need to identify clinical factors associated with post-treatment efficacy of galcanezumab, which could assist clinicians and patients when selecting the best treatment option and treatment duration for the individual.
The objectives of this analysis of the Japanese randomized controlled clinical trial and open-label extension were to evaluate efficacy after discontinuation of galcanezumab, both in patients with EM and in patients with CM, and to identify clinical factors associated with changes in monthly migraine headache days after discontinuation of galcanezumab.
Methods
Study Design
The primary trial was a 6-month, randomized, double-blind, placebo-controlled study of galcanezumab in Japanese people with EM (ClinicalTrials.gov, NCT02959177), conducted across 40 sites from December 2016 to January 2019 [9]. The open-label extension study was a 12-month safety study of galcanezumab in Japanese people with either EM or CM (ClinicalTrials.gov, NCT02959190), conducted at 44 sites from March 2017 to August 2019 [16]. The protocols for both trials were reviewed and approved by local ethics review boards, and written informed consent was obtained from all patients before participation. Both studies were conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. A list of the institutional ethics review boards is provided in Table S1.
Study Populations and Treatment Protocols
The inclusion and exclusion criteria for both trials have been published previously [9, 16]. Briefly, patients eligible for the primary trial were adults with a migraine diagnosis, had migraine for ≥ 1 year prior to the study, and had 4–14 migraine headache days and ≥ 2 migraine attacks per month (i.e., EM) [9]. Patients eligible for the open-label extension trial included those with EM who completed the treatment period of the primary trial and newly recruited patients with CM. Patients with CM were eligible if they were adults with a migraine diagnosis, had ≥ 1 headache-free day per month in the 3 months prior to treatment and during the baseline period, and had ≥ 15 headache days per month (of which ≥ 8 had features of migraine) during the baseline period [16].
In the primary trial, patients were randomized (2:1:1) to monthly subcutaneous injections of placebo, 120 mg of galcanezumab, or 240 mg of galcanezumab [9]. Patients randomized to galcanezumab 120 mg received a single 240-mg loading dose at the start of treatment [9]. Patients with EM who received galcanezumab 120 mg or 240 mg in the primary trial remained on the same dose in the open-label extension study [16]. Patients with EM who received placebo in the primary trial and patients with CM who were newly recruited were randomized (1:1) to galcanezumab 120 mg or 240 mg in the open-label extension study [16]. All patients randomized to galcanezumab 120 mg received a single 240-mg loading dose at the start of open-label treatment [16]. In the primary trial, all injections were performed by trained study personnel [9]; in the open-label study, after 6 months, patients were given the option to self-administer the injection under the supervision of study personnel [16].
This analysis included three patient groups. The first group of patients, from the primary trial, had EM, received 6 months of treatment with either placebo or galcanezumab, and were followed up for 4 months post-treatment. The second group of patients had EM, rolled over to the open-label extension study from the primary trial, received 12 months of open-label treatment with galcanezumab, and were followed up for 4 months post-treatment. Patients who rolled over into the open-label extension trial from the primary trial were not included in the post-treatment follow-up of the primary trial. In this group, patients who received placebo in the primary trial received a total of 12 months of galcanezumab treatment, and patients who received galcanezumab in the primary trial received a total of 18 months of galcanezumab treatment. The third group of patients comprised newly recruited patients with CM who entered the extension study, received 12 months of open-label galcanezumab treatment, and were followed up for 4 months post-treatment.
Outcome Measures
The primary and key secondary outcomes of both the primary and open-label extension trials have been reported previously [9, 16]. In this prespecified analysis, the efficacy outcome was the number of monthly migraine headache days during the post-treatment phase after discontinuation of galcanezumab. A migraine headache day was defined as a calendar day on which a migraine or probable migraine headache occurred. Each month was defined as a 30-day period with migraine or headache measures, normalized from the intervals between visits. Participants in both studies used daily migraine diaries to record the frequency of headaches, migraine headaches, and medications used for headache during the treatment and post-treatment phases.
Prespecified safety assessments included frequency of adverse events (AEs), serious AEs, treatment-emergent AEs (TEAEs), and post-TEAEs (PTEAEs). A PTEAE was defined as an event that first occurred or worsened during the post-treatment phase when compared with baseline. In this analysis, we report safety during the post-treatment phase of the primary trial; safety during the post-treatment phase of the open-label study has been reported previously [16]. AEs were coded using the Medical Dictionary for Regulatory Activities Version 21.1.
This analysis of data from the primary and extension trials also includes a post hoc exploratory analysis to identify clinical factors of migraine associated with changes in monthly migraine headache days during the post-treatment phases of both trials.
Statistical Analysis
The efficacy analysis population consisted of patients in the intent-to-treat (ITT) population of the primary trial who entered the post-treatment phase of the trial and who had migraine headache day data recorded both (1) at the final month of the treatment period and (2) at the end of the post-treatment period. The ITT population of the primary trial was defined as all randomized patients who received ≥ 1 dose of placebo or galcanezumab [9]. The efficacy population of the open-label extension trial was the same as the primary trial [16].
Baseline (pre-treatment) demographic and clinical characteristics are reported as mean (SD) for continuous variables and n (%) for categorical variables, for each of the treatment groups and for 50% responders and non-responders to galcanezumab. In each population, a 50% responder to galcanezumab was defined as any patient who had a ≥ 50% reduction in the total number of migraine headache days (relative to baseline) during the last month of the treatment period.
Clinical factors associated with changes in monthly migraine headache days during post-treatment phases were determined using a three-step process. The clinical factor variables used in the post hoc analyses are listed in Table S2. Firstly, Hall’s method [17] was used to select important (continuous) covariates with a high correlation with the response variable, but lower correlations among the selected covariates. Here the response variable was the change in migraine headache days during the post-treatment phase (migraine headache days in the last month of the follow-up period minus migraine headache days in the last month of the treatment period). This was based on Eq. 4.16 in [17]. In the original algorithm, the selection stops at the maximum point in the merit function Ms. Because we had a next step to eliminate variables, we wanted to avoid too strict a selection in this step. Therefore, we stopped the selection when the merit function decreased slightly (0.01 decrease) after reaching the maximum point (in Ms) as the number of selected covariates (k in Eq. 4.16) increased. We used the non-parametric method, Kendall’s correlation in the Hall’s algorithm. Secondly, a random forest procedure [18] was used to refine the explanatory variables selected by Hall’s method, plus one binary variable sex (R package randomForest, “randomForest” function). The response variable was the same as the Hall’s method. Thirdly, after constructing 500 random forest trees, we computed important scores by decreases in mean squared errors (MSE). We selected variables with ≥ 30% of the maximum decrease in MSE among the input variables (R package randomForest, “varImpPlot” function). Note that there is no standard cutoff to select variables, so we used the relatively conservative cutoff of 30%. Finally, linear regression without interaction terms was used with the variables selected in the previous steps. As a cutoff value, we used a p value < 0.05. This process was conducted separately for all primary trial patients who received galcanezumab, all extension trial patients with EM, all extension trial patients with CM, 50% responders in the primary trial, 50% responders with EM in the extension trial, and 50% responders with CM in the extension trial. For all these post hoc analyses, there was no multiplicity adjustment.
Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R, Version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) [19].
Results
Patient Disposition and Baseline Characteristics
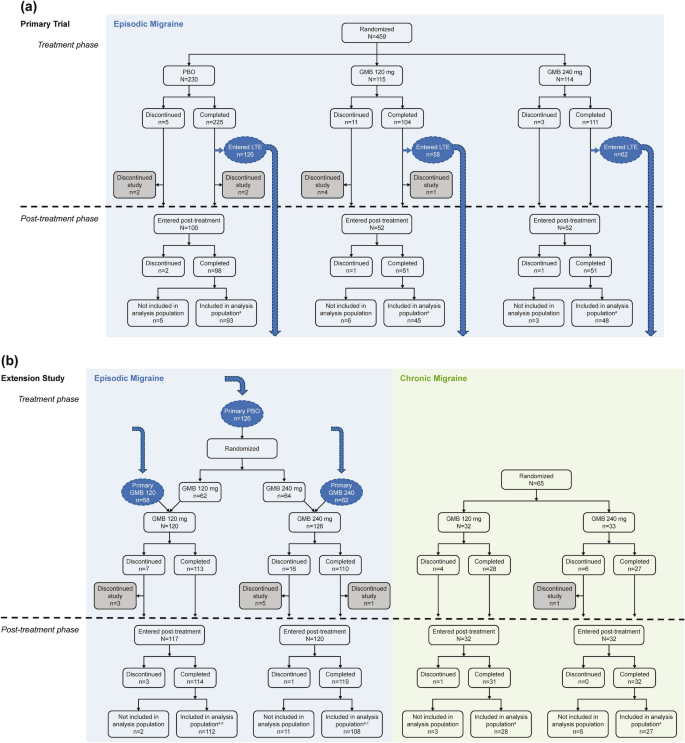
As previously described for the primary trial [9], 459 patients with EM were randomized to placebo (N = 230), galcanezumab 120 mg (N = 115), and galcanezumab 240 mg (N = 114) (Fig. 1). Some patients who completed the treatment period in the primary trial shifted into the open-label extension trial and thus were not included in the post-treatment population of the primary trial. This included 58 patients on galcanezumab 120 mg and 62 patients on galcanezumab 240 mg who continued on the same dose in the extension trial, and 126 patients who received placebo in the primary trial and were re-randomized to galcanezumab 120 mg (n = 62) or galcanezumab 240 mg (n = 64) in the extension trial. Thus, in the primary trial, 204 patients entered the post-treatment phase (placebo N = 100; galcanezumab 120 mg N = 52; galcanezumab 240 mg N = 52), and 186 patients completed the post-treatment phase and were included in the analysis population (placebo N = 93; galcanezumab 120 mg N = 45; galcanezumab 240 mg N = 48). In the extension trial, 237 patients with EM entered the post-treatment phase (galcanezumab 120 mg N = 117; galcanezumab 240 mg N = 120), and 220 patients completed the post-treatment phase and were included in the analysis population (galcanezumab 120 mg N = 112; galcanezumab 240 mg N = 108). The disposition of patients with EM in the analysis population by primary trial dose and extension trial dose was 57 patients receiving placebo then galcanezumab 120 mg, 55 receiving placebo then galcanezumab 240 mg, 55 receiving galcanezumab 120 mg then 120 mg, and 53 receiving galcanezumab 240 mg then 240 mg (Table 1).
Patient flow for the primary and extension trials, including patients who transferred from the primary trial into the open-label extension study. aPatients included in the analysis population had migraine headache days recorded for the last month of treatment and the last month of the post-treatment phase. bDisposition by primary trial dose/extension trial dose: PBO/GMB120 N = 57; GMB120/GMB120 N = 55. cDisposition by primary trial dose/extension trial dose: PBO/GMB240 N = 55; GMB240/GMB240 N = 53. GMB galcanezumab, LTE long-term extension, PBO placebo
Patients with CM were also included in the extension trial (N = 65) and randomized to galcanezumab 120 mg (N = 32) or 240 mg (N = 33). Of these, 64 patients entered the post-treatment phase (galcanezumab 120 mg N = 32; galcanezumab 240 mg N = 32), and 55 patients completed the post-treatment phase and were included in the analysis population (galcanezumab 120 mg N = 28; galcanezumab 240 mg N = 27).
Demographic and clinical characteristics were mostly well balanced across treatment groups in the analysis population (Table 1). The percentage of female patients was around 82–85% in most treatment groups, although 90.9% of the placebo/galcanezumab 240-mg group with EM and 100% of the galcanezumab 120-mg group with CM in the extension trial were female. Some expected differences were seen between patients with EM and those with CM. In patients with EM, the percentage who had previously failed ≥ 1 preventive migraine treatment ranged from 28.9% to 50.9%, whereas it was slightly higher in patients with CM (55.6–64.3%; consistent with previous reports for Japanese patients with CM [3]).
The demographic and clinical characteristics among 50% responders to galcanezumab in each trial were mostly similar across the trials (Table S3).
Efficacy After Discontinuation of Galcanezumab
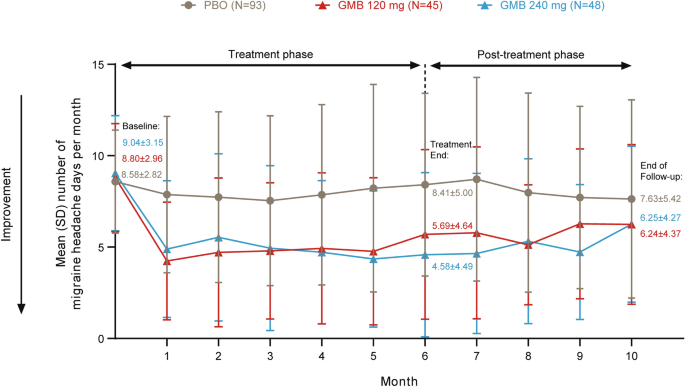
In patients with EM who received 6 months of treatment with galcanezumab in the primary trial, the number of monthly migraine headache days increased during the 4-month post-treatment phase compared with the end of the treatment period but remained lower than baseline (Fig. 2). Patients in the galcanezumab 120-mg group experienced a mean (SD) of 5.69 (4.64) monthly migraine headache days in the final month of treatment (month 6) and 6.24 (4.37) monthly migraine headache days at the end of follow-up (month 10) compared with 8.80 (2.96) at baseline (before treatment). In the galcanezumab 240-mg group, mean monthly migraine headache days were 9.04 (3.15) at baseline (before treatment), 4.58 (4.49) at month 6 (end of treatment), and 6.25 (4.27) at month 10 (end of follow-up).
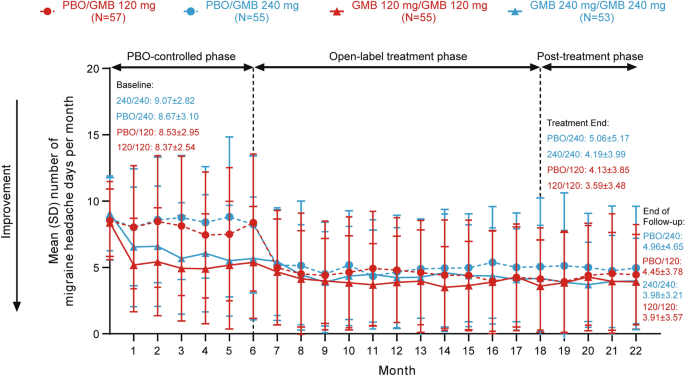
After 12 or 18 months of galcanezumab treatment, patients with EM maintained a similar frequency of monthly migraine headache days during the 4-month post-treatment phase and during treatment (Fig. 3). In patients who received placebo for 6 months followed by galcanezumab 120 mg for 12 months, the mean (SD) number of monthly migraine headache days was 4.13 (3.85) in the final month of treatment and 4.45 (3.78) at the end of follow-up compared with 8.53 (2.95) at baseline (before treatment). Patients who received galcanezumab 120 mg for 18 months had mean (SD) monthly migraine headache days of 8.37 (2.54) at baseline (before treatment), 3.59 (3.48) at month 18 (end of treatment), and 3.91 (3.57) at the end of follow-up. In patients who received placebo for 6 months followed by galcanezumab 240 mg for 12 months, the mean (SD) number of monthly migraine headache days was 5.06 (5.17) in the final month of treatment and 4.96 (4.65) at the end of follow-up compared with 8.67 (3.10) at baseline (before treatment). Patients who received galcanezumab 240 mg for 18 months had mean monthly migraine headache days of 9.07 (2.82) at baseline (before treatment), 4.19 (3.99) at month 18 (end of treatment), and 3.98 (3.21) at the end of follow-up.
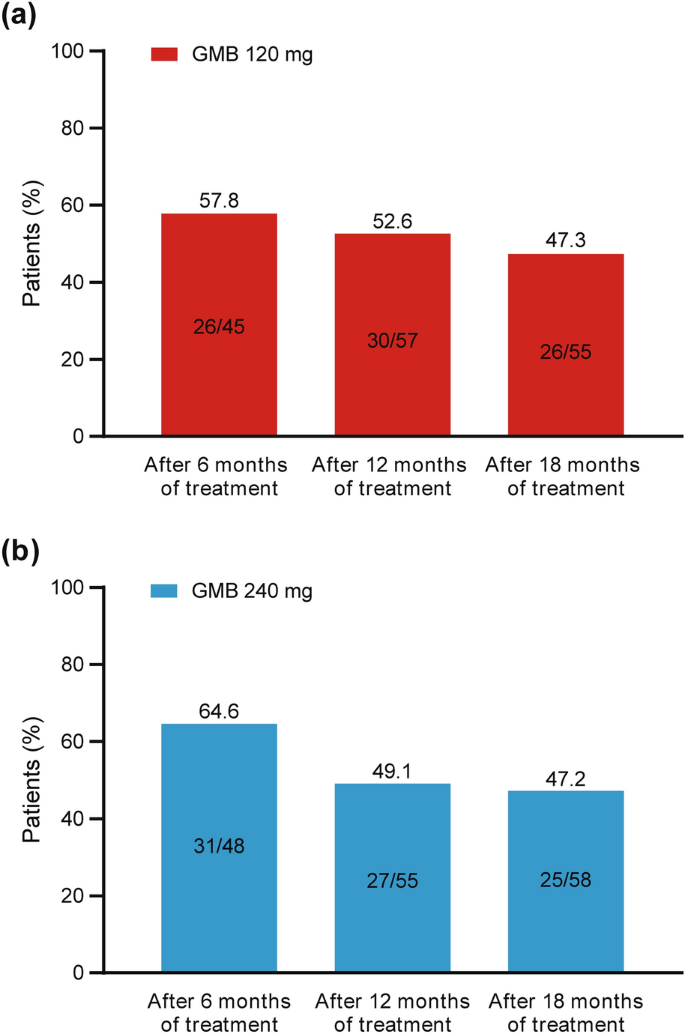
By pooling the data for patients with EM from the primary and extension trials, we were able to visualize the change in migraine headache days from the end of treatment to the end of the post-treatment phase across treatment durations and galcanezumab dose groups (Table 2). There was a numerical trend toward a smaller change in monthly migraine headache days after treatment discontinuation as the treatment duration increased. Furthermore, the percentage of patients with EM who experienced an increase in monthly migraine headache days (i.e., a worsening of their condition) during the post-treatment phase was greatest for patients with the shortest treatment period (Fig. 4). For patients receiving galcanezumab 120 mg, the percentage who experienced a worsening of their condition during the post-treatment phase was 57.8% (26/45) after 6 months, 52.6% (30/57) after 12 months, and 47.3% (26/55) after 18 months of treatment. For patients receiving galcanezumab 240 mg, the percentage who experienced a worsening of their condition during the post-treatment phase was 64.6% (31/48) after 6 months, 49.1% (27/55) after 12 months, and 47.2% (25/58) after 18 months of treatment.
Percentage of patients with EM who experienced an increase in monthly migraine headache days during the post-treatment phase (i.e., worsened condition after the end of treatment). Data for 6 months of treatment are from the primary trial and data for 12 and 18 months of treatment are from the extension trial. EM episodic migraine, GMB galcanezumab
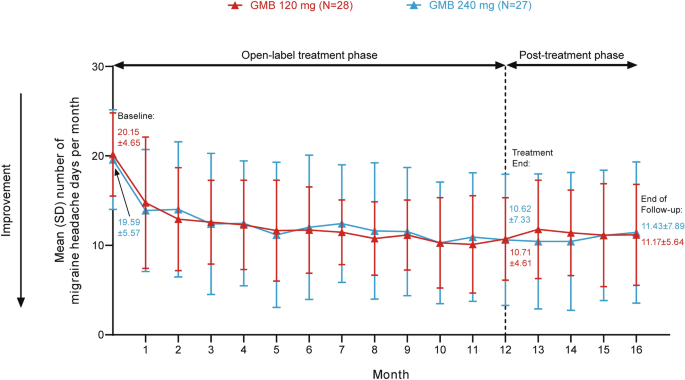
Patients with CM who received 12 months of galcanezumab treatment also demonstrated continued efficacy of galcanezumab for 4 months following treatment, with almost no change in migraine headache days during the follow-up period (Fig. 5). Patients receiving galcanezumab 120 mg had a mean (SD) baseline of 20.15 (4.65) monthly migraine headache days (before treatment), which decreased to 10.71 (4.61) at the end of treatment and was 11.17 (5.64) at the end of the 4-month follow-up. For patients in the galcanezumab 240-mg group, monthly migraine headache days were 10.62 (7.33) at the end of treatment and 11.43 (7.89) at the end of follow-up compared with 19.59 (5.57) at baseline (before treatment).
Safety After Discontinuation of Galcanezumab
No deaths were reported during the follow-up period for the primary trial. The only serious AE reported during the post-treatment phase occurred in one patient in the placebo group (moderate pneumonia, considered by the investigator as unrelated to the study drug). In the galcanezumab 240-mg group, there was a single discontinuation from the study during the post-treatment phase due to AEs. Overall, 30 patients (30%) in the placebo group, 21 patients (40.4%) in the galcanezumab 120-mg group, and 20 patients (38.5%) in the galcanezumab 240-mg group reported ≥ 1 PTEAE. The PTEAEs that occurred in ≥ 2% of patients who received any dose of galcanezumab were nasopharyngitis (7.7% of each of the 120-mg and 240-mg dose groups), back pain (1.9% and 3.9% of the 120-mg and 240-mg groups, respectively), gastroenteritis (5.8% and 0% of the 120-mg and 240-mg groups, respectively), influenza (3.9% and 1.9% of the 120-mg and 240-mg groups, respectively), migraine without aura (0% and 3.9% of the 120-mg and 240-mg groups, respectively), and oral herpes (3.9% and 0% of the 120-mg and 240-mg groups, respectively).
AEs that occurred during the post-treatment phase of the open-label extension study have been previously reported [16].
Clinical Factors Associated with Efficacy After Discontinuation of Galcanezumab
For the primary trial data (patients with EM after 6 months of treatment), after the random forest selection procedure, the variables selected were headache days per month at baseline (HD), migraine headache days per month at baseline (MHD), years since migraine diagnosis (DIAGyear), age, number of migraine headache days in the final month of treatment (BASEend), and the percentage change in monthly migraine headache days at treatment end (PCHGend) (Table S4). In the linear regression with no interaction terms, an increase in monthly migraine headache days after discontinuation of galcanezumab was significantly associated with a lower number of headache days per month at baseline (HD variable, p = 0.012) and longer disease duration (DIAGyear variable, p = 0.040) (Table 3).
In patients with EM in the extension trial (after 12 or 18 months of treatment), the variables selected for linear regression were the number of days with aura per 30-day period at baseline (AURA), number of migraine headache days in the final month of treatment (BASEend), and the percentage change in monthly migraine headache days at treatment end (PCHGend) (Table S5). An increase in monthly migraine headache days after discontinuation of galcanezumab was significantly associated with a higher number of days with aura per 30-day period (at baseline) (AURA variable, p = 0.004) and a lower number of monthly migraine headache days at treatment end (BASEend variable, p < 0.001) (Table 3).
In patients with CM in the extension trial (after 12 months of treatment), the variables selected by the random forest procedure were headache days per 30-day period at baseline (HD), number of days with acute medication use per 30-day period (NUMMU), body mass index at baseline (BMI), number of migraine headache days in the final month of treatment (BASEend), and the percentage change in migraine headache days at treatment end (PCHGend) (Table S6). However, in the linear regression, none of these clinical factors were significantly associated with an increase in monthly migraine headache days after discontinuation of treatment.
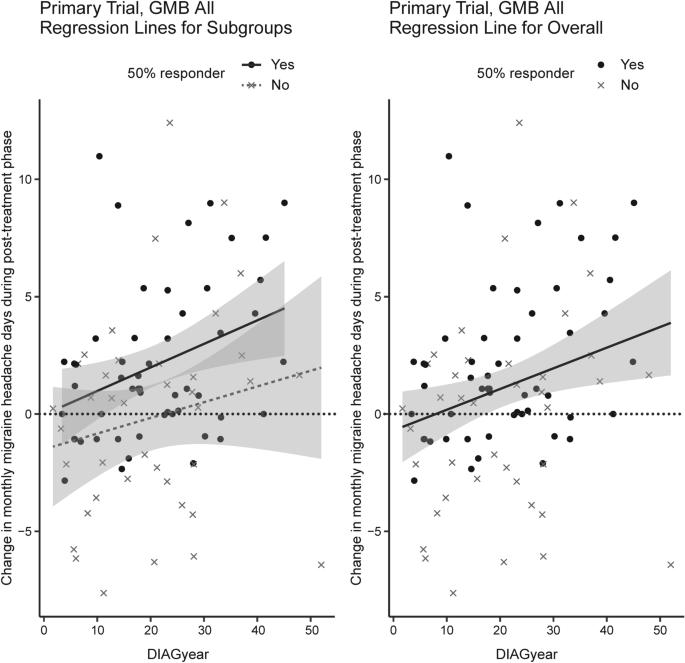
When the same analyses were performed on the 50% responder groups, only the patients with EM after 12 or 18 months of treatment showed any clinical factors significantly associated with changes in monthly migraine headache days after discontinuation of galcanezumab (Table 3). Specifically, in the 50% responder group, an increase in monthly migraine headache days after galcanezumab discontinuation was associated with the number of monthly migraine headache days with aura at baseline (AURA variable, p = 0.044) and a higher percentage change in monthly migraine headache days at treatment end (PCHGend variable, p = 0.012). A similar, although not statistically significant, trend was seen in the relationship between changes in monthly headache days and disease duration (Fig. 6).
Discussion
This analysis of data from both a randomized controlled trial and an open-label extension study of galcanezumab in Japanese patients with migraine provides the first description of post-treatment effects of galcanezumab in this population. After 12 or 18 months of treatment with galcanezumab, Japanese people with EM and with CM maintained reduced monthly migraine headache days for 4 months. This confirms previous reports from global studies of a post-treatment effect of galcanezumab in people with migraine [12]. This analysis also identified clinical factors associated with changes in migraine headache days after discontinuation of galcanezumab. These results may assist clinicians in identifying patients who may be likely to experience prolonged post-treatment efficacy. The demonstrated post-treatment effect of galcanezumab is also important for patients with migraine who may need to temporarily cease preventive medication or switch to another medication.
In the primary trial, the number of monthly migraine headache days increased slightly during the post-treatment phase but did not return to baseline levels in the galcanezumab-treated patients with EM. This pattern was seen in patients receiving both 120 mg and 240 mg of galcanezumab. This is consistent with the results of the EVOLVE-1 and -2 global phase 3 clinical trials of galcanezumab in patients with EM [12]. In both EVOLVE-1 and -2, the reduction in monthly migraine headache days declined during the post-treatment phase, and the number of monthly migraine headache days remained significantly different from baseline for all treatments at all time points [12]. In the open-label extension study, in both patients with EM and patients with CM, the number of monthly migraine headache days during the post-treatment phase remained significantly different from baseline in patients receiving galcanezumab, with very little change in migraine headache days from end of treatment to the end of the follow-up period. A prolonged difference from baseline monthly migraine headache days was reported in the 4-month period after 9 months of open-label extension of the REGAIN global clinical trial of galcanezumab in patients with CM [20].
The post-treatment effects of galcanezumab observed in the current analysis are generally consistent with previous reports for other migraine medications targeting CGRP or its receptor, although direct comparisons between studies are difficult owing to differences in methodology, duration of post-treatment period, and reported patient outcomes. For example, in patients with EM and with CM in a real-world clinical setting, there was a significant reduction in monthly migraine days compared with baseline during weeks 1–4 after discontinuation of erenumab [13]. Post-treatment effects have also been demonstrated in a small cohort study of patients with CM who were treated with erenumab or galcanezumab after previous failure of ≥ 3 preventive treatments [14]. In these patients, a significant reduction in monthly migraine days compared with baseline was observed at 1, 2, and 3 months post-discontinuation, although with a significant increase at 2 and 3 months compared with the last month of treatment [14].
In the present study, a numerical trend was observed of a smaller change in monthly migraine headache days as the treatment duration increased (in patients with EM). After 6 months of treatment and 4 months of follow-up, migraine headache days increased by 0.55–1.68 days per month from the end of treatment; after 12 or 18 months of treatment, and 4 months of follow-up the change was − 0.21 to + 0.32 days per month, depending on galcanezumab dose. We also observed that the percentage of patients with EM who experienced an increase in monthly migraine headache days during the post-treatment phase was greatest for patients treated for 6 months, and was relatively similar for patients who received 12 or 18 months of treatment. It is therefore likely that a longer treatment period may be associated with a prolonged post-treatment effect and a lower proportion of patients with a worsened condition.
In this analysis, we identified clinical factors associated with changes in monthly migraine headache days after discontinuation of galcanezumab by a random forest procedure and subsequently by linear regression without interaction effects. Although some variables appeared to have a similar clinical meaning, we kept them in the random forest and linear regression analyses because our first step (Hall’s algorithm) would have eliminated highly correlated non-important variables (e.g., HD and MHD, BASEend and PCHGend, for the primary trial analysis).
After 6 months of treatment with galcanezumab in patients with EM, random forest analysis identified the variables of headache days per month at baseline, migraine headache days per month at baseline, age, migraine headache days in the final month of treatment, percentage change in monthly migraine headache days at treatment end, and disease duration (years since diagnosis) as being associated with changes in monthly migraine headache days during the post-treatment phase. In the linear regression analysis, an increase in monthly migraine headache days during the post-treatment phase was only associated with a lower number of headache days per month at baseline and longer disease duration. It is possible that the association with longer disease duration is related to central sensitization, which is known to be associated with longer disease duration [21]; 6 months of treatment may be insufficient for patients with a longer disease duration to develop improved central sensitization.
After 12 or 18 months of treatment with galcanezumab in patients with EM, changes in monthly migraine headache days in the post-treatment phase were associated in the random forest analysis with the number of migraine headache days with aura at baseline, the number of migraine headache days in the final month of treatment, and the percentage change in monthly migraine headache days at treatment end. The linear regression identified a significant association of increasing number of monthly migraine headache days post-treatment with a higher number of migraine headache days per month with aura (at baseline) and a lower number of migraine headache days in the final month of treatment. It is possible that patients with a lower number of monthly migraine headache days at the end of treatment experienced an increase in migraine headache days during the post-treatment phase simply because the lower headache frequency at treatment end could not be maintained without additional doses of galcanezumab. It is also possible that the post-treatment effect of galcanezumab may not persist in patients with more frequent baseline aura because galcanezumab acts primarily peripherally, whereas the primary cause of aura is thought to be a central mechanism (depolarization within the cerebral cortex [22]). It is not clear why the clinical factors associated with post-treatment changes in monthly migraine headache days differed depending on the treatment period, and further research is needed.
For patients with CM, after 12 months of treatment, changes in monthly migraine headache days in the post-treatment phase were associated in the random forest analysis with headache days per month at baseline, the number of days per month with acute medication use, BMI, the number of migraine headache days in the final month of treatment, and the percentage change in monthly migraine headache days at treatment end. However, there were no significant associations between the change in monthly migraine headache days post-treatment and any variables in the linear regression.
The safety profile of galcanezumab during the post-treatment phase in Japanese patients with migraine was consistent with previous local and global studies. PTEAEs in the primary trial were reported by 30–40% of patients, consistent with the open-label extension trial [16]. The most common PTEAE in the primary trial was nasopharyngitis, which was also the most commonly reported PTEAE in the open-label extension trial [16]. These safety parameters are similar to the global EVOLVE-1 and -2 trials, in which PTEAEs were reported in around 25% of patients, and the most common PTEAEs were upper respiratory tract infections [12].
Limitations
Limitations of this analysis include the small sample size for some treatment groups, especially the 50% responder groups, and that restrictions in enrollment criteria for the two trials may have limited the generalizability of the results [12]. Our analyses used data from company-sponsored clinical trials and may not reflect the situation for patients in real-world clinical settings. There were no patients who received placebo treatment throughout the open-label extension, and so we were unable to investigate any impact of placebo responses on the results of the open-label extension trial, including in patients with CM. Participants were permitted to use preventive medication during the post-galcanezumab treatment period, which could have affected the results; however, only a minority of participants (5.9–7.7% of patients with EM; 23.4% of patients with CM) actually took such medication. A further potential limitation is that the post-treatment duration was 4 months, and our results may not be generalizable for longer durations; however, we note that the post-treatment duration was no shorter than previous post-treatment studies [12,13,14]. Investigating in more detail the variables selected by the random forest procedure may be a future analysis approach because the random forest algorithm can account for interaction effects that were omitted by the simple linear regressions in the last step of our analysis. We also note that in our analyses, patients were considered to have worsened if they had any increase in monthly migraine headache days post-treatment, even if this was only one migraine day per month; in practice, patients may not consider a small increase to be a worsened outcome, although this cannot be confirmed without patient-reported satisfaction or quality-of-life data. The strengths of our analysis included the use of data from two robustly conducted studies: one randomized, placebo-controlled, multicenter phase 2 trial and one randomized, multicenter, open-label extension study. Both the primary and extension trials included a prolonged post-treatment phase (4 months) in the study design in order to assess post-treatment effects described in this analysis. In addition, this is the first study to evaluate post-treatment effectiveness of galcanezumab in Japanese people with migraine, and the study population included patients both with EM and with CM.
Conclusion
Galcanezumab exhibited post-treatment efficacy for up to 4 months in Japanese patients with EM and with CM. Clinical factors that were associated with post-treatment increases in monthly migraine headache days in patients with EM included fewer headache days per month at baseline, longer disease duration, higher aura frequency at baseline, and a higher percentage change in monthly migraine headache days at treatment end.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the Eli Lilly repository at www.vivli.org. Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9–160.
Meyers JL, Davis KL, Lenz RA, Sakai F, Xue F. Treatment patterns and characteristics of patients with migraine in Japan: a retrospective analysis of health insurance claims data. Cephalalgia. 2019;39(12):1518–34.
Ueda K, Ye W, Lombard L, et al. Real-world treatment patterns and patient-reported outcomes in episodic and chronic migraine in Japan: analysis of data from the Adelphi migraine disease specific programme. J Headache Pain. 2019;20(1):68.
Vécsei L, Majláth Z, Szok D, Csáti A, Tajti J. Drug safety and tolerability in prophylactic migraine treatment. Expert Opin Drug Saf. 2015;14(5):667–81.
Durham PL. CGRP-receptor antagonists–a fresh approach to migraine therapy? N Engl J Med. 2004;350(11):1073–5.
Dodick DW, Goadsby PJ, Spierings ELH, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13(9):885–92.
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–8.
Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–54.
Sakai F, Ozeki A, Skljarevski V. Efficacy and safety of galcanezumab for prevention of migraine headache in Japanese patients with episodic migraine: a phase 2 randomized controlled clinical trial. Cephalalgia Rep. 2020;3:1–10.
Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–21.
Mulleners WM, Kim BK, Láinez MJA, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2020;19(10):814–25.
Stauffer VL, Wang S, Voulgaropoulos M, Skljarevski V, Kovacik A, Aurora SK. Effect of galcanezumab following treatment cessation in patients with migraine: results from 2 randomized phase 3 trials. Headache. 2019;59(6):834–47.
De Matteis E, Affaitati G, Frattale I, et al. Early outcomes of migraine after erenumab discontinuation: data from a real-life setting. Neurol Sci. 2021;42(8):3297–303.
Iannone LF, Fattori D, Benemei S, Chiarugi A, Geppetti P, De Cesaris F. Predictors of sustained response and effects of the discontinuation of anti-calcitonin gene related peptide antibodies and reinitiation in resistant chronic migraine. Eur J Neurol. 2022;29(5):1505–13.
Vernieri F, Brunelli N, Guerzoni S, et al. Retreating migraine patients in the second year with monoclonal antibodies anti-CGRP pathway: the multicenter prospective cohort RE-DO study. J Neurol. 2023;270(11):5436–48.
Hirata K, Takeshima T, Sakai F, et al. A long-term open-label safety study of galcanezumab in Japanese patients with migraine. Expert Opin Drug Saf. 2021;20(6):721–33.
Hall MA. Correlation-based feature selection for machine learning [PhD thesis]. Hamilton, New Zealand: The University of Waikato; 1999.
Breiman L. Random forests. Mach Learn. 2001;45(1):5–32.
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019.
Kuruppu DK, North JM, Kovacik AJ, Dong Y, Pearlman EM, Hutchinson SL. Onset, maintenance, and cessation of effect of galcanezumab for prevention of migraine: a narrative review of three randomized placebo-controlled trials. Adv Ther. 2021;38(3):1614–26.
Güven H, Çilliler AE, Çomoğlu SS. Cutaneous allodynia in patients with episodic migraine. Neurol Sci. 2013;34(8):1397–402.
Fraser CL, Hepschke JL, Jenkins B, Prasad S. Migraine aura: pathophysiology, mimics, and treatment options. Semin Neurol. 2019;39(6):739–48.
Acknowledgements
The authors would like to thank all study participants.
Medical Writing/Editorial Assistance.
Medical writing assistance was provided by Koa Webster, PhD, CMPP, and Rebecca Lew, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by Eli Lilly Japan K.K. and Daiichi Sankyo Company, Limited. ProScribe’s services complied with international guidelines for Good Publication Practice.
Funding
This study was funded by Eli Lilly Japan K.K., Kobe, Japan, manufacturer/licensee of galcanezumab in Japan. Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis, and preparation of the manuscript. The study sponsor also funded the journal’s Rapid Service and Open Access Fees.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mika Komori, Akichika Ozeki, and Yuka Tanji; Funding acquisition and supervision: Mika Komori; Project administration: Mika Komori and Yuka Tanji; Data curation, formal analysis, investigation, methodology, software, validation, and visualization: Akichika Ozeki; Writing—review and editing: Takao Takeshima, Hikaru Doi, Satomi Ooba, Yuka Tanji, Akichika Ozeki, and Mika Komori.
Corresponding author
Ethics declarations
Conflict of Interest
Takao Takeshima received research funding/collaborative research expenses from Biohaven, Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Lundbeck Japan K.K., and Shionogi & Co., Ltd., and reports personal fees from Amgen K.K., Daiichi Sankyo Company, Limited, Eli Lilly Japan K.K., and Otsuka Pharmaceutical Co., Ltd. Takao Takeshima also acted as an advisor to Hedgehog MedTech, Inc. and Sawai Pharmaceutical Co., Ltd. Hikaru Doi acted as an advisor to Amgen K.K. and Otsuka Pharmaceutical Co., Ltd.; reports research funding from AbbVie GK, Allergan, Inc., Amgen K.K., Biohaven, Ltd., Eli Lilly Japan K.K., Lundbeck Japan K.K., Otsuka Pharmaceutical Co., Ltd., and Pfizer Japan Inc.; and received personal fees from Amgen K.K., Daiichi Sankyo Company, Limited, Eli Lilly Japan K.K., and Otsuka Pharmaceutical Co., Ltd. Satomi Ooba reports personal fees from Amgen K.K., Daiichi Sankyo Company, Limited, Eli Lilly Japan K.K., and Otsuka Pharmaceutical Co., Ltd. Yuka Tanji, Akichika Ozeki, and Mika Komori are employees of Eli Lilly Japan K.K. and are shareholders in Eli Lilly and Company.
Ethical Approval
The protocols for both trials were reviewed and approved by local ethics review boards, and written informed consent was obtained from all patients before participation. Both studies were conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. A list of the institutional ethics review boards is provided in Table S1.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Takeshima, T., Doi, H., Ooba, S. et al. Clinical Evaluation After Discontinuation of Galcanezumab in Japanese Patients with Episodic and Chronic Migraine: Analysis of a Randomized, Placebo-Controlled Trial and Open-label Extension Study. Neurol Ther 13, 697–714 (2024). https://doi.org/10.1007/s40120-024-00602-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-024-00602-z