Abstract
Introduction
Eptinezumab is a humanized IgG1 immunoglobulin monoclonal antibody administered intravenously as a preventative migraine treatment. Previously conducted randomized, double-blind, placebo-controlled trials exhibited significant reductions in monthly migraine frequency among adults experiencing episodic and chronic migraine. The present study seeks to expand upon the current findings and to evaluate eptinezumab’s efficacy as a preventative treatment for chronic and episodic migraine patients in the United Arab Emirates. This study is intended to represent the first real-world evidence and will hopefully serve as a valuable complement to the existing literature on the subject.
Methods
This was a retrospective exploratory study. The participants included within the study were adult (≥ 18 years) patients diagnosed with either episodic or chronic migraine. Patients were categorized according to their history of previous preventative treatment failure. For the final assessment of treatment efficacy, we included only patients with a minimum of 6 months of clinical follow-up data. Patients were assessed at baseline for their monthly migraine frequency and assessed again at months 3 and 6. The primary objective was to evaluate the efficacy of eptinezumab in reducing migraine frequency among chronic and episodic migraine patients.
Results
A total of 100 participants were identified, of whom 53 completed the study protocol at month 6. Of the total, 40 (75.47%) were female, 46 (86.79%) were Emirati locals, and 16 (30.19%) were pharmaceutically naïve, having never tried any prior preventative therapy. Additionally, 25 (47.17%) patients met the criteria for chronic migraine (CM), whereas the remaining 28 (52.83%) were diagnosed with episodic migraine (EM). The baseline monthly migraine frequency (MMD) was 12.23 (4.97) days across all participants, 15.56 (3.97) for CM patients, and 9.25 (3.76) for EM patients; by month 6, these frequencies reduced to 3.66 (4.21), 4.76 (5.32), and 2.68 (2.61), respectively. Overall, 58.49% of those enrolled experienced > 75% reduction in MMD frequency by month 6.
Conclusion
Patients enrolled in this trial experienced clinically significant reductions in MMD by month 6. Eptinezumab was well tolerated and with one AE of significance that led to discontinuation from the study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Migraine is experienced by a significant large global population and is responsible for a huge burden and reduced quality of life in those affected. |
Recent approaches to migraine treatment have utilized anti-CGRP monoclonal antibodies; early findings are promising, but existing literature is scarce, particularly among underreported populations in clinical research and real-world setting. |
What did the study ask? |
This current real-world setting, retrospective study sought to investigate the effectiveness of intravenously administered eptinezumab in reducing chronic and episodic patients’ monthly migraine days (MMDs). |
What were the study outcomes/conclusions? |
On average, patients experienced significant reductions in MMDs by 6 months of follow up and the medication and was well-tolerated. |
What was learned from the study? |
Eptinezumab represents a novel and promising migraine treatment strategy; however, long-term follow-up data is warranted to understand the effect of dosage escalation and PPTF history on the effectiveness and tolerability. |
Introduction
Migraine is a neurological disorder that is experienced by an estimated 14.0% of the global population. It is a highly prevalent condition that can have a significant impact on an individual's quality of life. The impact of migraines on an individual's life can range from irritating to debilitating, depending on the severity of the attacks and on the individual's ability to manage their symptoms [1]. Therefore, given the significant impact that migraines can have on individuals' quality of life, it is indeed imperative that adequate research is devoted to improving our understanding of the pathophysiology and treatment of this condition [2]. Prevailing belief regarding the onset of a migraine attributes the pain to neural vasodilation within intracranial tissue, caused, in part, by the release of calcitonin gene-related peptide (CGRP) [3]. CGRP is prevalent in the trigeminal ganglion and plays an active role in nociception [4].
Recent attempts at anti-migraine medication specifically target CGRP receptors; such is the case with eptinezumab, approved by the FDA in 2020 [5]. Eptinezumab is a humanized IgG1 immunoglobulin monoclonal antibody (-mAb) administered intravenously to prevent migraines [6], and acts by binding to α-CGRP and β-CGRP.
Eptinezumab’s status as the first intravenous CGRP-mab offers its potential advantages over similar medications of the same class, due to its high binding affinity and improved dosage precision, and direct administration into the bloodstream allowing for 100% bioavailability. Through PREVAIL, a 2-year, open-label, placebo-controlled study, 128 adults suffering from chronic migraine were administered 300 mg intravenously every 3 months for up to 8 doses, and were then followed for 5 months. One month following the first injection, 61.10% of patients reported perceived improvement of condition; this portion improved to 83.30% 5 months after the final injection [7].
A similar study, entitled PROMISE-2, was conducted in 2020. This was a phase 3, randomized, double-blind, placebo-controlled study designed to assess the viability of eptinezumab as a preventative migraine treatment strategy [8]. Participants in the study received intravenous administration of eptinezumab of either 100-mg or 300 mg-doses 12 weeks apart, and were observed over the course of 32 weeks beginning at screening. Both doses significantly decreased migraine frequency when compared to placebo. Furthermore, these benefits were heightened following the administration of the second dose, possibly suggesting compounding effects. For this study, as well as its predecessor PROMISE-1, participants were excluded if experiencing confounding pain syndromes or untreated psychiatric disorder, or had received recent monoclonal antibody treatment or botulinum toxin injection [9].
This current study offers insight into the potential use of eptinezumab in a real-world setting as a preventative migraine treatment. In the United Arab Emirates (UAE), eptinezumab is available and fully covered for all Emirati nationals at no cost, and partially covered for non-Emirati residents with copayment, which ranges between 20 and 30%. However, there remain gaps in our knowledge regarding the long-term safety implications, as well as how this treatment method may differentially impact diverse populations in the real-world setting. Furthermore, the focus on a primarily Arab population makes for unique evidence of pharmaceutical treatment on an underreported population in pivotal trials. Despite possessing a prevalence falling within the global estimated range, there remains a lack of empirical research assessing migraine treatment strategies among Arab populations [10].
Methods
Study Design
This was a retrospective, single-site exploratory study. The participants included in the study were adult (≥ 18 years) patients diagnosed with either episodic or chronic migraine, as per the International Classification of Headache Disorders (ICHD-3) criteria.
For the purposes of this study, recruited patients were categorized according to their previous preventative treatment failure (PPTF), or the lack thereof. These patients had previously reported unsatisfactory or ineffective results on CGRP-mabs or other oral preventative treatments and opted to switch to eptinezumab as an alternative. Patients without any PPTF were considered treatment-naïve. It is important to note that the term “naïve” refers to individuals who have not tried other anti-CGRP-based therapies or any standard of care oral treatments for migraine prevention. Across participants, the most common PPFTs were galcanezumab and erenumab. All participating patients were prescribed an initial eptinezumab dose of 100 mg following baseline assessment; however, this dose was subject to increase to 300 mg at the physician's discretion according to the individual needs of the patient. Baseline MMD, and all other patient demographics, were assessed and recorded during the initial clinical visit, prior to administration of the first eptinezumab dose. After thorough discussion with their treating neurologists, patients agreed to begin or switch to eptinezumab as a migraine treatment strategy, and agreed to return every 3 months for each additional dose and follow-up assessment.
Demographic information, diagnosis, medication history, and baseline MMD were derived from clinical records. Participants’ perception of treatment effectiveness was similarly documented in clinical records, as well as in their ‘headache diaries’, where they were asked to document their symptoms post-treatment. Patient headache diaries were kept and logged from baseline, throughout treatment duration, and at subsequent follow-up visits. Treatment efficacy was determined according to the change in MMD between assessments. Treatment safety was determined according to the presence or absence of adverse events (AE).
Patients were assessed at their baseline frequency of MMDs and subsequently administered an initial 100-mg dose of eptinezumab. They were then reassessed and administered additional doses at both 3 months and 6 months following the initial assessment. Patients not completing the study protocol through 6 months at the time of data analysis were not included in the final analysis. The occurrence of AEs was documented and considered when evaluating the safety and tolerability of eptinezumab-jjmr.
Ethics
The research was conducted in accordance with the Declaration of Helsinki and consistent with GCP and the applicable regulatory requirements. The American Center for Psychiatry and Neurology Institutional Review Board waived the need for informed consent for this study as it involved a retrospective analysis. All relevant ethical, health authority, data privacy, and site-specific approvals were obtained prior to the start of the study. According to the site’s local Institutional Review Board regulations, a waiver of informed consent from the corresponding ethics committee was obtained. All authors were given access to the study data.
Sample
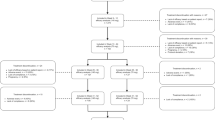
The patient identification process, as shown in Fig. 1, was conducted with precision, where 100 patients were identified for potential inclusion in data analysis. Each patient was carefully screened and identified based on the administration of at least two doses of eptinezumab. A total of 53 patients completed their 6-month follow-up and thus were included in the effectiveness analysis. Patients were excluded from the effectiveness analysis if they had not yet reached the 6-month follow-up. The excluded 47 patients were still on treatment but had not reached the 6-month follow-up point; hence, their exclusion was not because of discontinuation due to lack of efficacy.
Objectives
The primary objective was to investigate the efficacy and safety of intravenous eptinezumab as a preventative migraine treatment. The aim was to examine possible differences in outcome for patients diagnosed with chronic and episodic migraine.
As a secondary end point, the differences in treatment efficacy and safety for patients with at least one PPTF were investigated. Therefore, in addition to treatment-naïve patients, participants who had previously been prescribed galcanezumab or erenumab were included in the analysis of outcomes.
Statistical Analysis
Descriptive statistics include the following demographic and clinical information: sex, nationality, age, age of migraine onset, PPTF, and diagnosis (CM or EM). Treatment efficacy was assessed according to patient testimonials, as per headache diaries, of MMD change from baseline since receiving treatment. Moreover, given this study’s focus on preventative care, concomitant acute medications were not investigated.
The following analysis is exploratory in nature and is not made in reference to any specific hypotheses. Numerical variables such as MMD have been reported as means and standard deviations (SD), whereas categorical variables have been reported using frequency and percentages. Paired, two-tailed t tests were conducted to analyze differences in mean MMD between clinical assessments. As a retrospective study, sample size calculation was not necessary as the study population was based on availability. No data is missing from this study.
Results
Demographics and Baseline Characteristics
Of the 100 patients, all but one are still on treatment at the time of this submission. However, 53 patients only met the inclusion criteria and are included in the final analysis. The descriptive statistics are shown in Table 1. Within the population pool, 25 (47.17%) patients met the criteria for a CM diagnosis, whereas the remaining 28 (52.83%) were diagnosed with EM. The mean (SD) age of participants was 36.42 (10.55) years, whereas the mean age of onset for migraine was 29.43 (8.74) years; these means did not differ significantly between CM and EM patients.
A key feature of this study’s population was an overwhelming majority of female (75.47%) and Emirati participants (86.79%). Among the 53 patients included in the effectiveness analysis, 35 (66.04%) had a history of 1 PPTF and 2 patients (3.77%) had 2 PPTFs. Overall, distribution of PPTF history was relatively even, with naive, erenumab, and galcanezumab patients accounting for 30.19%, 30.19%, and 32.08%, respectively. Among the remaining 7.55%, 4 participants had previously tried other preventative therapies, 2 were previously prescribed flunarizine, a calcium antagonist, while the other 2 had a history of 2 PPTFs; both had previously been prescribed erenumab and galcanezumab. Among the 53 patients who had completed the study protocol through month 6, 14 (26.42%) had undergone a dose increase, due to inadequate response, from 100 to 300 mg at some point after the initiation of treatment. In this case, inadequate response refers to less than 50% reduction in MMDs. Of the 14 patients, 8 met the criteria for a CM diagnosis.
The mean (SD) baseline frequency of MMD across all participants was 12.23 (4.97). When examining CM and EM data individually, MMD baseline frequency diverges to 15.56 (3.97) and 9.25 (3.76), respectively. The mean MMD frequencies of CM and EM patients differed significantly (p < 0.001).
Chronic Migraine and Episodic Migraine
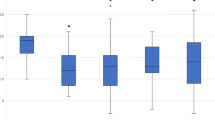
Across all patients, the mean (SD) MMD frequency decreased from 12.23 (4.97) to 5.21 (5.73) by month 3 (M3), and 3.66 (4.21) by month 6 (M6). For specifically CM patients, mean MMD decreased from 15.56 (3.97) at baseline (BL) to 7.76 (6.78) by (M3), and 4.76 (5.32) by M6. For EM patients, mean BL MMD decreased from 9.25 (3.76) to 2.93 (3.30) by M3 and 2.68 (2.61) by M6. Across all patients, the decrease in MMD from BL to M3 (p < 0.001) and BL to M6 (p < 0.001) was statistically significant, as was the decrease from M3 to M6 (p = 0.02). This trend also holds when independently analyzing CM and EM patients. For the CM patients, a paired, two-tailed t test yielded a statistically significant difference for the change from BL to M3 (p < 0.001) and BL to M6 (p < 0.001) and for M3 to M6 (p = 0.03). Similarly, for EM patients a t est yielded a statistically significant difference for the change from BL to M3 (p < 0.001) and BL to M6 (p < 0.001), but not for M3 to M6 (p = 0.745). These findings are shown in Fig. 2.
As shown in Fig. 3, 58.49% of patients experienced at least a 75% reduction in their MMD frequency by month 6. Furthermore, only 15.09% of patients experienced less than a 50% reduction in MMD. A similar proportion of patients achieved > 75% reduction among CM (60.00%) and EM patients (57.14%). CM patients appeared to be less responsive initially (32.00% achieving > 75% reduction by month 3) when compared to EM patients (57.14%).
Previous Preventative Treatment Failure
As part of this study’s exploratory objectives, patients were categorized according to their PPTF history, or lack thereof. As set out in Table 2, mean comparison tests were conducted between naïve patients and those with PPTF history across the following variables: age, age of migraine onset, baseline MMD frequency, M3 MMD frequency, and M6 MMD frequency. Naïve and PPTF patients did not differ significantly in mean age (p = 0.174) nor age of migraine onset (p = 0.702). Additionally, naïve and PPTF patients did not differ significantly in baseline MMD frequency (p = 0.881). However, at M3, naïve patients (mean = 2.13, SD = 1.86) had significantly lower mean MMD frequency (p < 0.001) than PPTF patients (mean = 6.61, SD = 6.36). Full descriptive statistics and t test results are presented in Table 2.
Exposure and Safety
Among the initial recruitment population (n = 100), three patients reported an AEs, only one of whom was excluded from the data analysis as treatment was discontinued. The most common AEs were itchiness/skin irritation (2.0%) and tears/blurred vision (2.0%). The excluded patient reported worsening headaches, increased photophobia, and a pimple-like rash. As a result, they were discontinued from the study. The remaining two patients experienced minor AEs during infusion; however, these were quickly resolved and did not impact their ability to continue treatment. One patient experienced itchiness, rhinorrhea, and teary eyes that was resolved after 10 min of infusion. The second patient experienced brief blurred vision which was similarly resolved after 10 min under the attending physician’s supervision. Both patients were discharged in stable condition and were able to continue the follow-up treatments without reoccurrence of AEs. Eptinezumab was well-tolerated across the remaining participants.
Discussion
To our knowledge, this study represents the first real-world evidence study of intravenous migraine treatment and the first study examining the efficacy and tolerability of intravenous migraine medication among chronic and episodic migraine patients in the UAE. Similar to our study, other real-world data on other mAbs have validated our understanding of the effectiveness of these therapies [11, 12]. Furthermore, the current study included diverse pools of patients in terms of their prior preventive therapies’ exposure in their treatment; the inclusion of naïve patients, as well as those with PPTF for migraine headache, allows for possible insights into how eptinezumab’s efficacy may be impacted by prior medication history. Our findings align strongly with those presented in PROMISE-1, PROMISE-2, and PREVAIL, in which the administration of eptinezumab significantly decreased migraine frequency with sustained effects over a 12-week period and was well tolerated by most patients. Our results suggest that additional doses of treatment may result in further reductions in MMDs in a subset of patients and an increased proportion of patients experiencing a > 75% in MMDs from baseline by week 24, which mirrors the findings presented in PROMISE-2.
A major objective was to assess potential differences in response to eptinezumab for chronic and episodic migraine patients. In line with standard diagnostic criteria, CM patients expressed a significantly greater frequency of MMD at baseline assessment. Furthermore, it is worth noting that EM patients exhibited greater improvements by month 3 when compared to CM counterparts. By month 3, 57.14% of EM patients had experienced a > 75% reduction in MMD, whereas only 32.00% of CM patients achieved similar success. Nevertheless, this disparity had approximately equalized by month 6. These findings, similar to those from other mAb studies, could indicate a greater treatment resistance to eptinezumab in CM patients, and can also be explained by possible greater CGRP levels on average when compared to EM patients. However, this outcome warrants further investigation into the safety and tolerability of 300-mg doses as a treatment strategy for CM or otherwise treatment-resistant patients. In this present study, 14 participants, 8 of whom were diagnosed with CM, agreed to an increase in dosage from 100 to 300 mg and no AEs were documented as a result. Among patients agreeing to a dosage increase, 4 experienced < 25% reductions in MMD, 1 experienced a 25–50% reduction, 6 patients showed a 50–75% reduction, while the remaining 3 patients experienced > 75% reduction in MMD by month 6.
It is possible that a previous prescription may impact the efficacy of eptinezumab. Our findings suggest that naïve patients are more responsive by month 3 than patients who had previously been prescribed galcanezumab and erenumab. Whether or not this means that they are resistant to CGRP-based therapy, future trials may provide alternative and probably better pathophysiological-based explanations.
Developing resistance towards treatment could be another possible explanation; however, this is not a common occurrence. A systematic review which was conducted to evaluate the treatment of resistant chronic migraine with anti-CGRP monoclonal antibodies mentions that developing tachyphylaxis is possible in a few cases, which could be due to an increased development of antibodies in response to treatment [13].
On the other hand, another study was conducted to investigate the development of anti-drug antibodies (ADAs). Based on their clinical immunogenicity evaluation, the results showed that developing ADAs was rare; however, the presence of ADAs had no effect on the efficacy or safety [14]. Clinical studies with eptinezumab have reported the highest rates of ADAs and NAbs in comparison to other CGRP inhibitors [15]. This observation is worth noting, knowing that eptinezumab is administered intravenously, while other CGRP mAbs are administered subcutaneously. Several factors influence immunogenicity and mitigation, including dosage, route of administration, and protein structure, as well as patient-related factors, such as concomitant medication use and comorbidities [16]. Future studies could help expand our knowledge to understand the long-term effects of the immunogenicity of eptinezumab.
Patients were asked and encouraged to keep a regular diary of their symptoms. However, MMD, the metric by which treatment efficacy is determined, is still reliant on a patient's testimony and their willingness to comply with filling out the diaries. Therefore, the accuracy of MMD is subject to the patient’s ability to recall the frequency of migraine attacks over, at least, the last month. As a result, naïve patients may have their perception skewed towards greater improvement due to the lack of a previous reference point for medication effectiveness, or patients tend to neglect accurate documentation of their daily headaches on a regular basis.
Our results have demonstrated the promise of intravenous medication as an effective treatment strategy for migraine. Given that the means of administration necessitates a clinician, it encourages consistent follow-ups with a healthcare provider and minimizes the risk of medication overuse/misuse. Most notably, most of the participants reported sustained improvements that spanned up to 3-month intervals, minimizing the burden of a daily prescription.
Regarding safety, administration of eptinezumab was well tolerated and all but one participant proceeded without adverse effects. For the aforementioned patient, they reported worsening headaches, increased photophobia, and a pimple-like rash, and discontinued from the study.
Future follow-up studies would benefit from the inclusion of further surveys intended to quantify the physical and affective impact of eptinezumab on the quality of the lives of patients with migraines. Surveys assessing participants’ monthly migraine/headache days, as well as changes in their emotional well-being and productivity, would allow for a more robust picture of eptinezumab’s impact on the lives of migraine patients.
Limitations
We acknowledge the limitations of the study as being retrospective in design and having a small sample size. Given that the patients were included in data analysis after outcomes had already taken place, selection bias risk is a possible barrier to generalizability. Similarly, the small cohort and 6-month usage minimum leave questions regarding the generalizability of the findings to long-term eptinezumab use.
Conclusions
This retrospective study examining the efficacy of eptinezumab among migraine patients in the UAE resulted in several valuable insights. Namely, early impressions suggest that intravenous migraine medication has the potential to be an effective and well-tolerated treatment strategy, which appears to be especially true for naïve patients, and for those diagnosed with episodic migraine.
Across participants, administration of 100 mg of eptinezumab appeared to result in at least moderate reductions in migraine frequency and severity, with sustained effects over the course of 3 months. However, a small portion of participants did not experience meaningful reductions in migraine symptoms until the dosage was increased to 300 mg. The relationship between dosage and efficacy remains a point of interest; the initial findings serve as a necessary starting point into further explorations pertaining to this association.
Overall, the results provide compelling evidence towards the possible use of a novel treatment strategy in a clinical context.
References
Stovner LJ, Hagen K, Linde M, Steiner TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. 2022;23:34.
Mungoven TJ, Henderson LA, Meylakh N. Chronic migraine pathophysiology and treatment: a review of current perspectives. Front Pain Res. 2021;2:705276.
Schoenen J, Manise M, Nonis R, Gérard P, Timmermans G. Monoclonal antibodies blocking CGRP transmission: an update on their added value in migraine prevention. Rev Neurol (Paris). 2020;176:788–803.
Bo Y, Li J, Wang Y. The mechanism of eptinezumab-jjmr targeting CGRP in the treatment of migraine. In: 2022 International Conference on Biotechnology, Life Science and Medical Engineering (BLSME 2022). Clausius Scientific Press; 2022.
Eptinezumab DS. First approval. Drugs. 2020;80:733–9.
Vijayanandh M. VYEPTI (eptinezumab-jjmr) intravenous drug as a preventive treatment of migraine. Pondicherry J Nurs. 2020;12:56–7.
Kudrow D, Cady RK, Allan B, Pederson SM, Hirman J, Mehta LR, et al. Long-term safety and tolerability of eptinezumab in patients with chronic migraine: a 2-year, open-label, phase 3 trial. BMC Neurol. 2021;21:126.
Yuan H, Silberstein SD. Eptinezumab. In: van den Brink AM, Martelletti P, editors. Monoclonal antibodies in headache: from bench to patient. Springer International Publishing; 2021. p. 109–19.
Ashina M, McAllister P, Cady R, Hirman J, Ettrup A. Efficacy and safety of eptinezumab in patients with migraine and self-reported aura: Post hoc analysis of PROMISE-1 and PROMISE-2. Cephalalgia. 2022;42:696–704.
El-Metwally A, Toivola P, AlAhmary K, Bahkali S, AlKhathaami A, Al Ammar SA, et al. The epidemiology of migraine headache in Arab countries: a systematic review. Sci World J. 2020;2020:1–11.
Alsaadi T, Noori S, Varakian R, Youssef S, Almadani A. Real-world experience of erenumab in patients with chronic or episodic migraine in the UAE. BMC Neurol. 2022;22:221.
Straube A, Stude P, Gaul C, Schuh K, Koch M. Real-world evidence data on the monoclonal antibody erenumab in migraine prevention: perspectives of treating physicians in Germany. J Headache Pain. 2021;22:133.
Sevivas H, Fresco P. Treatment of resistant chronic migraine with anti-CGRP monoclonal antibodies: a systematic review. Eur J Med Res. 2022;27:86.
Pederson S, Biondi DM, Allan B, Cady R, Schaeffler B, Baker B, et al. Clinical immunogenicity evaluation of eptinezumab, a therapeutic humanized monoclonal antibody targeting calcitonin gene-related peptide (CGRP) for the preventive treatment of migraine. Front Immunol. 2021;12:765822.
Cohen JM, Ning X, Kessler Y, Rasamoelisolo M, Campos VR, Seminerio MJ, et al. Immunogenicity of biologic therapies for migraine: a review of current evidence. J Headache Pain. 2021;22:3.
Chirmule N, Jawa V, Meibohm B. Immunogenicity to Therapeutic Proteins: Impact on PK/PD and Efficacy. AAPS J. 2012;14:296–302.
Acknowledgements
Funding
The study received no funding. The rapid service was funded by the authors.
Author Contributions
Yazan Bader helped collecting data and analyzing results, and Medline search. Ibrahim Al Qaisi, Mohamad Harb, Vanessa Santos helped collecting data. Reem Suliman, Taoufik Alsaadi helped interpreting data, write up and overseeing the project. All authors read and approved the final manuscript.
Disclosures
Yazan Bader, Reem Suliman, Ibrahim Al Qaisi, and Vanessa Santos, Mohamad Harb and Taoufik Alsaadi have no competing interests and nothing to disclose.
Compliance with Ethics Guidelines
The research was conducted in accordance with the Declaration of Helsinki and consistent with GCP and the applicable regulatory requirements. The American Center for Psychiatry and Neurology (ACPN) Institutional Review Board has waived the need for informed consent for this study as it involved a retrospective analysis.
Data Availability
All data generated or analyzed during this study are included in this published article. No data repository is available.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bader, Y., Suliman, R., Harb, M. et al. Effectiveness and Safety of Eptinezumab in Episodic and Chronic Migraine Headache in the UAE: A Retrospective Study. Neurol Ther 12, 1683–1693 (2023). https://doi.org/10.1007/s40120-023-00521-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00521-5