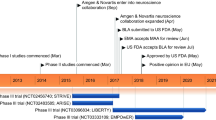
Abstract
Eptinezumab-jjmr (referred to as eptinezumab hereafter; Vyepti™) is a humanised monoclonal antibody that binds to calcitonin gene-related peptide (CGRP) and blocks its binding to the receptor. CGRP is believed to play a major role in the pathophysiology of migraine. Eptinezumab, delivered by intravenous (IV) administration, is being developed by Lundbeck Seattle BioPharmaceuticals for the prevention of migraine. In February 2020, eptinezumab was approved in the USA for the preventive treatment of migraine in adults. This article summarizes the milestones in the development of eptinezumab leading to this first approval.
Similar content being viewed by others
References
Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4(5):386–98.
Durham PL, Vause CV. Calcitonin gene-related peptide (CGRP) receptor antagonists in the treatment of migraine. CNS Drugs. 2010;24(7):539–48.
Lundbeck. Vyepti (eptinezumab-jjmr): US prescribing Information. 2020. https://www.lundbeck.com/upload/us/files/pdf/Products/Vyepti_PI_US_EN.pdf. Accessed 5 Mar 2020.
Lundbeck. FDA approves Lundbecks VYEPTI™ (eptinezumab-jjmr): the first and only intravenous preventive treatment for migraine [media release]. 2020. https://www.lundbeck.com.
Alder B. Alder BioPharmaceuticals® enters into European patent settlement and global license agreement with Teva in the field of anti-CGRP-based therapy [media release]. https://investor.alderbio.com/news-releases/news-release-details/alder-biopharmaceuticalsr-enters-european-patent-settlement-and. Accessed 8 Jan 2018.
Lundbeck AS. Lundbeck completes the acquisition of Alder BioPharmaceuticals—a company committed to transforming migraine treatment and prevention [media release]. https://www.lundbeck.com. Accessed 22 Oct 2019.
Data on file, Lundbeck Seattle Biopharmaceuticals, Inc. 2020.
Latham J, Karasek C, Ojala E, et al. Characterization of the intrinsic binding features of three anti-CGRP therapeutic antibodies effective in preventing migraine: a comparative case study of ALD403, Ly-2951742, TEV-48125 [abstract no. PS74LB]. Headache. 2016;56(8):1398.
Baker B, Schaeffler B, Beliveau M, et al. Population pharmacokinetics of eptinezumab for the preventive treatment of migraine [abstract no. IHC-DP-032]. Cephalalgia. 2019;39(1 Suppl):37.
Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020. https://doi.org/10.1177/0333102420905132.
Lipton RB, Saper J, Ashina M, et al. A phase 3 study to evaluate eptinezumab for the preventive treatment of chronic migraine: results of the PROMISE-2 (PRevention Of Migraine via Intravenous Eptinezumab Safety and Efficacy-2) trial [abstract no. PF02]. Headache. 2018;58(Suppl 2):80.
Kudrow D, Lipton R, Silberstein S, et al. Eptinezumab for prevention of chronic migraine: results of 2 infusions in the phase 3 PROMISE-2 (prevention of migraine via intravenous eptinezumab safety and efficacy-2) trial [abstract no. P2.10-006]. Neurology. 2019;92(15 Supplement):5.
Schim J, Goadsby P, Kassel E, et al. Eptinezumab increases days free from canonical migraine-associated symptoms within 1 month of treatment in patients with chronic Migraine [abstract no. P4.10-024]. Neurology. 2019;92(15 Suppl):20.
Cady R, McGill L, Hirman J, et al. Patient global impression of change related to improvement in most bothersome symptom following treatment with eptinezumab [abstract no. S38.009]. Neurology. 2019;92(15 Suppl):20.
Nagy AJ, Silberstein SD, Cady R, et al. Treatment with eptinezumab demonstrated meaningful improvements in patients with chronic migraine experiencing a high frequency of severe migraines [abstract no. P219LB]. Headache. 2019;59(Suppl 1):163.
Lipton RB, McGinley JS, Houts C, et al. Eptinezumab demonstrated early and sustained reductions in HIT-6 total score over time in patients with chronic migraine in the PROMISE-2 trial [abstract no. P215LB]. Headache. 2019;59(Suppl 1):159–60.
McGinley JS, Buse D, Wirth R, et al. Early and sustained reduction in headache impact in people with CM after eptinezumab treatment: HIT-6 item analysis in the phase 3 PROMISE-2 trial [abstract no. P216LB]. Headache. 2019;59(Suppl 1):160–1.
Lipton RB, McGinley JS, Hirman J, et al. HIT-6 responder rates after 1 and 3 months of eptinezumab treatment [abstract no. IHC-OR-018]. Cephalalgia. 2019;39(1 Suppl):12–3.
Winner P, McAllister P, Kassel E, et al. Eptinezumab reduces migraine frequency within the first month after treatment in patients with episodic or chronic migraine [abstract no. P1.10-023]. Neurology. 2019;92(15 Suppl):20.
Kudrow D, Diamond M, McGill L, et al. Eptinezumab achieved meaningful reductions in migraine activity as early as day 1:PROMISE-2 (prevention of migraine via intravenous eptinezumab safety and efficacy-2) phase 3 trial in chronic migraine [abstract no. IOR04]. Headache. 2018;58(Suppl 2):74
Winner PK, McAllister P, Cady R, et al. Migraine-free months in patients with episodic or chronic migraine treated with eptinezumab: results from the PROMISE-1 and PROMISE-2 trials [abstract no. P217LB]. Headache. 2019;59(Suppl 1):162.
Tepper SJ, Smith T, Kassel E, et al. Eptinezumab reduces the frequency of acute medication usage in patients with episodic or chronic migraine [abstract no. P218LB]. Headache. 2019;59(Suppl 1):162–3.
Chakhava G, Cady R, Kassel E, et al. Consistent reductions in migraine frequency with eptinezumab treatment in patients with migraine stratified by disease characteristics: subgroup analysis of PROMISE-1 and PROMISE-2 [abstract no. A42]. J Headache Pain. 2019;20(Suppl 1):20.
Janelidze M, Giorgadze G, Cady R, et al. Consistent reductions in migraine frequency with eptinezumab treatment in patients with migraine stratified by intrinsic factors: subgroup analyses of PROMISE-1 and PROMISE-2 [abstract no. A45]. J Headache Pain. 2019;20(Suppl 1):20.
Diamond M, Lipton R, Horblyuk R, et al. Early impact of Eptinezumab on the health-related quality of life (HRQoL) of patients with episodic or chronic Migraine: SF-36 analysis across the spectrum of migraine [abstract no. P1.10-025]. Neurology. 2019;92(15 Suppl):20.
Diamond M, Lipton RB, Horblyuk R, et al. Impact of eptinezumab on the health-related quality of life (HRQoL) of patients with episodic or chronic migraine: SF-36 analysis by months 1 and 3 across the spectrum of migraine [abstract no. PND82]. Value Health. 2019;22(Suppl 2):S285.
Tepper S, Kudrow D, Horblyuk R, et al. Early migraine response by month 1 and clinically meaningful improvements in health-related quality of life (HRQOL) in patients with migraine in phase 3 trials of eptinezumab [abstract no. P2.10-020]. Neurology. 2019;92(15 Suppl 1):20.
Spierings E, Biondi D, Hirman J, et al. Reduced impact of headaches after migraine preventive treatment with eptinezumab in patients with chronic migraine: results from the PREVAIL open-label safety study [abstract no. P16]. Headache. 2019;59(Suppl 1):36.
Kudrow D, Berman G, Kassel E, et al. Eptinezumab treatment for migraine prevention reduces migraine disability in patients with chronic migraine: an analysis from the PREVAIL open-label safety study [abstract no. P17]. Headache. 2019;59(Suppl 1):36–7.
Kassel E, Huffman C, Hirman J, et al. Eptinezumab improved health-related quality of life in patients with chronic migraine in the open-label PREVAIL study [abstract no. P138]. Headache. 2019;59(Suppl 1):112.
Allan B, Khan A, Song Y, et al. PREVAIL: an open-label phase 3 trial to evaluate the safety of eptinezumab administered intravenously in patients with chronic migraine [abstract no. P125]. Headache. 2019;59(Suppl 1):105.
Song Y, Biondi D, Cady R, et al. An open-label phase 3 trial to evaluate the safety of eptinezumab administered intravenously in patients with chronic migraine (PREVAIL): demographics and baseline characteristics [abstract no. P158]. Headache. 2019;59(Suppl 1):121–2.
Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic Migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13(11):1100–7.
Dodick DW, Lipton RB, Silberstein S, et al. Eptinezumab for prevention of chronic migraine: a randomized phase 2b clinical trial. Cephalalgia. 2019;39(9):1075–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. S. Dhillon is a contracted employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.12037707.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Dhillon, S. Eptinezumab: First Approval. Drugs 80, 733–739 (2020). https://doi.org/10.1007/s40265-020-01300-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01300-4