Abstract
Introduction
Current treatments for relapsing–remitting multiple sclerosis (RRMS) are only partially effective. The objective of this study was to characterize treatment response in RRMS patients in Portugal to 12-month therapy with first-line disease-modifying therapies.
Methods
In this retrospective study, neurologists at participating centers completed survey questionnaires using records of patients with RRMS who had received first-line treatment with one of five European Medicine Agency-approved agents in the 12 months prior to inclusion in the survey. Sub-optimal responders included patients treated for at least 1 year, and who had ≥1 relapse(s) or an increase of 1.5 points on the Expanded Disability Status Scale (EDSS; if baseline EDSS was 0) or an increase of ≥0.5 points (baseline EDSS ≥1). Optimal responders included patients treated for at least 1 year without relapse and who had an increase of <1.5 points on EDSS (if baseline EDSS was 0) or no increase in EDSS (baseline EDSS ≥1).
Results
Data for 1,131 patients from 15 centers were analyzed. Twenty-six percent (95% confidence interval 23–28%) of patients had sub-optimal treatment response. Duration of therapy (P < 0.001), age at the start of therapy (P = 0.03), and baseline EDSS score (P < 0.001), were significantly different among treatments. Sub-optimal treatment response appeared to be related only to a more severe EDSS score at baseline and did not differ among therapies.
Conclusion
Neurologists should closely monitor patients to optimize treatment strategies and better control disease, improving prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a debilitating disease, which is estimated to affect ~5,000 people in Portugal based on the crude prevalence of MS (46.3/100,000 inhabitants) found in the sole published study [1]. Despite the availability of different disease-modifying therapies (DMTs) to slow the progression of disability and reduce relapse rates of relapsing–remitting MS (RRMS), these therapies only show moderate effectiveness and in most patients the disease progresses [2–4]. Approximately, 62–75% of patients relapse after 2 years of disease and 20–27% have an increase in their disability of at least one point in the Expanded Disability Status Scale (EDSS) [5–9]. Close monitoring of response to first-line DMTs becomes important in identifying patients with sub-optimal response to improve treatment strategies and better control disease.
Therapeutic response is based on the evaluation of relapse rates, disability (as measured by EDSS) and magnetic resonance imaging (MRI) [10–12]. Relapse is a good indicator of clinical disease activity, however, its use as an isolated measure is controversial [10]. Changes in EDSS alone must also be regularly confirmed to overcome possible false or transient values associated with relapses or other medical conditions [10]. MRI has a high additional value in monitoring response to therapy, since inflammatory events occur more frequently than clinical events [13], but its usefulness per se in monitoring treatment response is not well established [10]. Several treatment response criteria based on these parameters have been proposed [14–18], although many were not validated over long-term follow-up periods [10], but there is no consensus about the best [10, 19]. Despite the lack of consensus, the potential of combining clinical (relapse rate and disability progression) and MRI measures, for predicting disease progression and monitoring treatment response, has been recognized [20].
Randomized, double-blind clinical trials are the “gold standard” for evaluating therapeutic efficacy, but observational studies might demonstrate whether the results from clinical trials are reflected in clinical practice, capturing variations in clinical evidence and generating new study hypotheses. The aim of this study was to characterize the types of treatment response to first-line DMTs in a “real-world” setting in Portuguese RRMS patients over 12 months.
Methods
Study Design
A retrospective study was performed in Portugal, between September 2008 and July 2009 to characterize a representative “real-world” sample of RRMS patients on treatment with DMTs for 12 months. A survey was used to capture data. This study design is an efficient method to obtain an updated national picture of treatment response to first-line DMTs in RRMS patients. Treatment response was defined either as optimal or sub-optimal (see Sect. “Treatment Response Criteria”), so that the association of treatment response with socio-demographic, clinical, and therapeutic factors could be studied.
Setting and Data Collection
Neurologists from 20 referral hospital centers for MS were invited to participate (one neurologist per center). Centers were categorized as large (>200 MS patients), medium (100–200 MS patients), and small volume (<100 MS patients) hospitals, according to the number of MS patients registered in each center. Each neurologist completed a survey questionnaire for RRMS patients at their center using medical records/charts. The neurologist also conducted a disease assessment of each patient at the time they completed the questionnaire. After site initiation, neurologists had 2 months to collect the data and fill the survey with the required information.
Confidentiality was secured using a coded identification for each patient. The Portuguese Committee of Data Protection approved the data collection required in this study, which was conducted according to current ethical and legal standards, and followed the Recommendations for Good Clinical Practice and ethical principles as stated in the Declaration of Helsinki of 1975, as revised in 2000 and 2008.
Participants and Eligibility Criteria
Patients with RRMS treated for at least 1 year with one specific DMT in the 12 months prior to questionnaire completion. The diagnostic criteria used for RRMS were those developed before 2008 (from Poser et al. [21] or McDonald et al. [22], depending on the year of diagnosis).
Data Collected in Questionnaire
The following data were captured in the questionnaire—patient characteristics: age, gender, and date of RRMS diagnosis; first-line DMT in the 12 months prior to the date of questionnaire completion (“study period”); whether the patient received such therapy or not, and if ‘yes’, the start and stop dates or if treatment was ongoing; and clinical disease history: disability assessed by the EDSS 12 months prior to the date of completion of the questionnaire (defined as baseline EDSS), number and date of any relapses in the prior 12 months, and disease duration.
The following were considered first-line therapies: interferon beta-1a intramuscular (IFNβ-1a IM), interferon beta-1b subcutaneous (IFNβ-1b SC), interferon beta-1a 22 μg SC (IFNβ-1a 22 μg SC), interferon beta-1a 44 μg SC (IFNβ-1a 44 μg SC), and glatiramer acetate SC (GA).
EDSS scores were collected from patient medical files. No estimations were allowed and so patients were excluded if any data were missing. EDSS scoring was performed by the treating MS neurologist. Neutralizing antibody testing was not undertaken as it is not routine clinical practice in Portugal.
Disability Assessment at Time Questionnaire Completed
EDSS was also assessed by the site neurologist at time the questionnaire was completed, allowing the measure of patient disability at the end of 12-month treatment.
Treatment Response Criteria
The treatment response criteria were the optimal and sub-optimal clinical responses. A sub-optimal clinical response was defined as occurring in patients receiving DMT for ≥1 year with one or more relapses, or patients treated with DMTs for ≥1 year with an increase by ≥1.5 points in EDSS (if baseline EDSS was 0), or by ≥0.5 points (if baseline EDSS was ≥1). An optimal clinical response was seen in patients treated with DMT for ≥1 year without relapses and, simultaneously, with an increase of <1.5 points in EDSS (if baseline EDSS was 0), or no increase in EDSS (if baseline EDSS was ≥1).
Study Size
The planned sample size was 1,200 patients with RRMS, representing ~35% of patients on treatment with DMTs in Portugal at the time [23].
Statistical Methods
Demographic and clinical characteristics of patients were analyzed through descriptive statistics and compared among therapies using one-way analysis of variance (ANOVA) or Fisher Exact tests for continuous or categorical variables, respectively. The non-parametric Kruskal–Wallis test was used to confirm the results obtained from ANOVA for variables with skewed distributions. Scheffé tests were used to determine which pairs of means were statistically different if ANOVA results were statistically significant. Patients’ socio-demographic characteristics were also compared between those with optimal and sub-optimal responses using two-sided t tests or Mann–Whitney tests for normally and non-normally distributed variables, respectively. Fisher Exact tests were used to assess the association between the various therapies and the type of response (sub-optimal vs. optimal). Univariate and multivariate logistic regression analyses were further used to investigate which socio-demographic and clinical characteristics were associated with the type of treatment response. Logarithmic transformations were used for variables with a skewed distribution. For all tests, a 5% level of significance was considered and the statistical software Stata SE version 10 (StataCorp LP, College Station, TX, USA) was used.
Results
Fifteen of the 20 invited neurology centers entered the study. Five centers were large, five were medium, and five were small. The remaining centers did not collect any patient data during the data collection period.
Participants
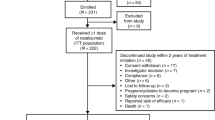
Neurologists completed survey questionnaires for 1,338 patients. We estimated that this sample size corresponds to 50% of the total number of patients in each hospital volume category, thus constituting a representative sample of the referral centers that follow and treat MS patients in Portugal. Of the 1,338 patients, 207 patients were excluded: 43 lacked information about the medication taken in the last 12 months, 39 received more than one DMT, 41 received DMTs not specified for inclusion in the survey, and 84 started therapy <1 year prior to survey completion. In total, a sample of 1,131 patients (84.5% of the eligible patients) was analyzed for their response to treatment with DMTs.
Descriptive Data
Most patients were female (68%); the mean age was 41 years, the mean age at diagnosis was 33 years and the mean disease duration was 8.3 years. Twenty percent of patients had relapses in the last 12 months, with an average of 0.3 relapses per patient in the 1-year period considered for this study; the median baseline EDSS score was 2.0 (Table 1).
Table 2 shows the proportion of patients taking each type of DMT and the respective treatment duration.
Socio-Demographic and Clinical Factors
Differences between treatment groups in socio-demographic and clinical characteristics are presented in Table 3. Duration of therapy, age at the start of therapy, and baseline EDSS differed significantly among treatments. Patients treated with GA had a significantly lower duration of therapy and were older than patients treated with other therapies. Baseline EDSS was significantly lower in patients treated with IFNβ-1a IM and IFNβ-1a 22 μg SC when compared with patients treated with other therapies.
Patterns of Treatment Response
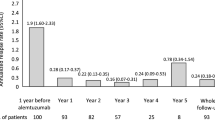
Twenty-six percent (95% CI 23–28%) of patients had sub-optimal response to treatment. The mean number of relapses, and baseline EDSS for the optimal responders were 0.0 and 2.0, respectively, and for the sub-optimal responders were 1.0 and 2.6, respectively. Percentages of sub-optimal and optimal responses by DMT are presented in Fig. 1. Treatment with IFNβ-1a 44 μg SC for 12 months appeared to be associated with a greater proportion of sub-optimal responses compared with the other therapies, in univariate analysis (Tables 3, 4); however, multiple logistic regression analysis showed that only EDSS at baseline was associated with a sub-optimal response to therapy (Table 4). Age, duration of therapy, gender, disease duration did not appear to influence response.
Discussion
The purpose of this survey was to characterize treatment response to first-line DMTs in RRMS patients in Portugal, and to identify the factors associated with treatment response. Our study was designed based on a retrospective multicenter study in Germany that included 461 centers and 8,275 patients [24].
The results of our study are in line with the previously mentioned German study. They showed that, despite treatment with DMTs, 20% of RRMS patients had relapses over a 12-month period, and 26% had sub-optimal treatment response. The proportion of patients with sub-optimal response was associated with worse baseline EDSS scores, and no differences in response were observed among therapies. A considerably high proportion of patients had one or more relapses (20%) indicating low disease control [25]. Results from previous studies showed that patients who present with any two of clinical disease activity, disease progression, or MRI activity after 12 months of therapy with IFNβ, are potentially eligible to switch therapies since the risk for disease progression is higher in subsequent years [10, 26].
The efficacy results shown in this study are consistent with an Italian independent study that found no between-treatment differences in the proportion of relapse-free patients and EDSS score changes at 12 months, in a cohort of 540 RRMS patients treated with different IFNβ therapies [27]. Furthermore, similar results were shown in another smaller retrospective cohort of 134 RRMS patients from Argentina, treated with IFNβ and GA for 16 months [28]. Although we found a higher percentage of relapse-free patients in patients receiving any IFNβ, GA relapse-free patient data in the Argentinian study is in line with this study. Similar to our results, Carrá et al. [28] did not find a significant reduction in EDSS scores in any of the treatment groups. Variability between baseline disease activity and treatment duration in each study population may explain such differences.
There is no consensus about the definition of optimal or sub-optimal response, but Rio et al. [15] evaluated the influence of different definitions on the treatment response to IFNβ treatment. The definition closest to ours considered the presence of one or more relapses, and an increase of 1.5 points for a baseline EDSS of 0 (1 point for EDSS between 1.0 and 5.0, and 0.5 points for EDSS ≥5.5) [15]. The number of relapses in the optimal responders group was similar to our study, but in the sub-optimal responders group, the average relapse rate was higher in the study of Rio et al. [15].This difference in relapse rate between studies may be due not only to the different definitions used for optimal and sub-optimal responder, but also to the duration of follow-up (2-year vs. 1-year follow-up in our study). In view of these results, it seems that treatment efficacy depends largely on the definition of response applied [15]. Indeed, in the literature, various factors are identified to predict response to therapy [14, 18, 27, 29], and these predictors vary greatly depending on the definition of response used [29].
This study has some limitations. Patient selection was not randomized, yet selection bias seems unlikely as ~50% of the total MS patient population from each hospital was included in this study. The retrospective nature of the study makes inferences of causality unfeasible; only possible associations can be ascertained. Possible associations between treatment and treatment response are confounded by use of different agents at different times in the disease course, even though the indications are essentially the same for all five agents. As the study was designed to evaluate therapies used in the 12 months preceding the questionnaire completion date, but no limitations were set on dates of treatment initiation when data were captured, some neurologists reported therapies that were administered before the 12-month period, supporting the idea that there may have been a carryover effect of these other treatments on response recorded for this study. Finally, the questionnaire did not capture the number of relapses before the 12-month period to ascertain whether there was an increase or decrease in the relapse rate or confirm the EDSS scores which could make the results of this analysis less robust.
Conclusion
This study makes an important contribution to the identification of types of treatment responses in patients with RRMS in Portugal, being unique in this sense. Given the fact that our study showed that almost one-third of patients are sub-optimal responders to therapy, it emphasizes the importance of physicians being alert to this possibility in their patients. Patients should be closely monitored to identify as early as possible RRMS cases with sub-optimal response to therapy. While we did not investigate how physicians in Portugal treated sub-optimal responders, physicians may consider switching therapies to avoid disease progression. Moreover, these switches should be implemented when it is still expected that other drugs are effective [7]. Our study draws attention to the fact that no major differences in type of response were found between treatments, but that all have a considerably high proportion of sub-optimal responses. The ability to identify patients with optimal or sub-optimal response to treatment is therefore important in that it may assist in the decision of what treatment to use and on the possibility of changing it.
References
De Sa J, Paulos A, Mendes H, Becho J, Marques J, Roxo J. The prevalence of multiple sclerosis in the District of Santarem. Portugal. J Neurol. 2006;253:914–8.
Sa MJ, Guimaraes J, Abreu P, Mendes A, Souto BE. Etiopathogenesis, classical immunotherapy and innovative nanotherapeutics for inflammatory neurological disorders. Curr Nanosci. 2011;7:2–20.
Mendes A, Sa MJ. Classical immunomodulatory therapy in multiple sclerosis: how it acts, how it works. Arq Neuropsiquiatr. 2011;69:536–43.
Sorensen PS. New management algorithms in multiple sclerosis. Curr Opin Neurol. 2014;27:246–59.
Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 1993;43:655–61.
Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. [Erratum appears in Lancet 1999;353(9153):678]. Lancet 1998;352:1498–504.
Galetta SL, Markowitz C, Lee AG. Immunomodulatory agents for the treatment of relapsing multiple sclerosis: a systematic review. Arch Intern Med. 2002;162:2161–9.
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). [Erratum appears in Ann Neurol 1996;40(3):480]. Ann Neurol 1996;39:285–94.
Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing–remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–76.
Rio J, Comabella M, Montalban X. Predicting responders to therapies for multiple sclerosis. Nat Rev Neurol. 2009;5:553–60.
Sormani MP, Rio J, Tintore M, et al. Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler. 2013;19:605–12.
Freedman MS, Selchen D, Arnold DL, et al. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can J Neurol Sci. 2013;40:307–23.
Isaac C, Li DK, Genton M, et al. Multiple sclerosis: a serial study using MRI in relapsing patients. Neurology. 1988;38:1511–5.
Rio J, Nos C, Tintore M, et al. Assessment of different treatment failure criteria in a cohort of relapsing–remitting multiple sclerosis patients treated with interferon beta: implications for clinical trials. Ann Neurol. 2002;52:400–6.
Rio J, Nos C, Tintore M, et al. Defining the response to interferon-beta in relapsing–remitting multiple sclerosis patients. Ann Neurol. 2006;59:344–52.
Rudick RA, Lee JC, Simon J, Ransohoff RM, Fisher E. Defining interferon beta response status in multiple sclerosis patients. Ann Neurol. 2004;56:548–55.
Villoslada P, Oksenberg JR, Rio J, Montalban X. Clinical characteristics of responders to interferon therapy for relapsing MS. Neurology. 2004;62:1653 (author reply).
Waubant E, Vukusic S, Gignoux L, et al. Clinical characteristics of responders to interferon therapy for relapsing MS. Neurology. 2003;61:184–9.
Rudick RA. Measuring the impact of therapeutic intervention. Neurology. 2010;74:S1–2.
Rio J, Castillo J, Rovira A, et al. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler. 2009;15:848–53.
Poser CM. Clinical diagnostic criteria in epidemiological studies of multiple sclerosis. Ann NY Acad Sci. 1965;122:506–19.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7.
Entidade Reguladora da Saúde (2007) Avaliação do acesso dos doentes com EM a consultas externas nos Hospitais do SNS. Available https://www.ers.pt/uploads/writer_file/document/101/824937_rel.pdf. Last accessed Aug 6, 2014.
Maurer M, Dachsel R, Domke S, et al. Health care situation of patients with relapsing–remitting multiple sclerosis receiving immunomodulatory therapy: a retrospective survey of more than 9000 German patients with MS. Eur J Neurol. 2011;18:1036–45.
Hutchinson M. Predicting and preventing the future: actively managing multiple sclerosis. Pract Neurol. 2009;9:133–43 (discussion 44).
Gajofatto A, Bacchetti P, Grimes B, High A, Waubant E. Switching first-line disease-modifying therapy after failure: impact on the course of relapsing–remitting multiple sclerosis. Mult Scler. 2009;15:50–8.
Trojano M, Liguori M, Paolicelli D, et al. Interferon beta in relapsing–remitting multiple sclerosis: an independent postmarketing study in southern Italy. Mult Scler. 2003;9:451–7.
Carra A, Onaha P, Sinay V, et al. A retrospective, observational study comparing the four available immunomodulatory treatments for relapsing–remitting multiple sclerosis. Eur J Neurol Off J Eur Fed Neurol Soc. 2003;10:671–6.
Portaccio E, Zipoli V, Siracusa G, Sorbi S, Amato MP. Response to interferon-beta therapy in relapsing–remitting multiple sclerosis: a comparison of different clinical criteria. Mult Scler. 2006;12:281–6.
Acknowledgments
This study was conceived and developed by the authors of this manuscript and was financed with a fair market honorary from Biogen Idec, Portugal. The article processing charges were funded by Biogen Idec. The authors received no other funding for this study. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. We thank Tracy Harrison and Mary Hines of inScience Communications, Springer Healthcare, for editorial assistance and styling of the manuscript prior to submission. This assistance was funded by Biogen Idec.
Conflict of interest
Maria José Sá has received research support from Bayer Schering, Bial, Biogen Idec, Fundação Schering Lusitana, Merck Serono, Sanofi, Schering-Plough, and Octapharma; speaker honoraria from Bayer Schering, Biogen Idec, Merck Serono, Novartis, and Sanofi; consultation fees on scientific advisory boards from Bayer Schering, Biogen Idec, CSL Behring, Merck Serono, and Novartis. João de Sá received consultation fees for participating on advisory boards sponsored by Biogen Idec, Ipsen Pharma, and Novartis Pharma, and honoraria as a speaker for participation in symposia for Teva, Novartis, and Biogen Idec. Lívia Sousa has received fees from Novartis Pharma, Biogen Idec, Merck Serono, Sanofi, Almirall, Bayer Schering, and Teva for participating on advisory boards or lecturers.
Compliance with ethics guidelines
The Portuguese Committee of Data Protection approved the data collection required in this study, which was conducted according to current ethical and legal standards, and followed the Recommendations for Good Clinical Practice and ethical principles as stated in the Declaration of Helsinki of 1975, as revised in 2000 and 2008.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sá, M.J., de Sá, J. & Sousa, L. Relapsing–Remitting Multiple Sclerosis: Patterns of Response to Disease-Modifying Therapies and Associated Factors: A National Survey. Neurol Ther 3, 89–99 (2014). https://doi.org/10.1007/s40120-014-0019-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-014-0019-4