Abstract
Background
Alemtuzumab is a high-efficacy treatment approved for relapsing-remitting multiple sclerosis (RRMS). Although clinical trials and observational studies are consistent in showing its efficacy and manageable safety profile, further studies under clinical practice conditions are needed to further support its clinical use.
Objective
The aim of this observational retrospective study was to evaluate the effectiveness and safety of alemtuzumab to add to the current real-world evidence on the drug.
Methods
A cohort of 115 adult patients with RRMS treated with alemtuzumab between 2014 and 2020 was retrospectively followed up in five centers in Spain. Analysis included annualized relapse rate (ARR), 6-month confirmed disability worsening (CDW), 6-month confirmed disability improvement (CDI), radiological activity, no evidence of disease activity (NEDA-3), and safety signals. Given the different follow-up periods among participants, ARR was calculated using the person-years method. CDI was defined as a ≥ 1.0-point decrease in Expanded Disability Status Scale (EDSS) score assessed in patients with a baseline EDSS score ≥ 2.0 confirmed 6 months apart. CDW was defined as a ≥ 1.0-point increase in EDSS score assessed in patients with a baseline EDSS score ≥ 1.0 (≥ 1.5 if baseline EDSS = 0), confirmed 6 months apart.
Results
ARR decreased from 1.9 (95% confidence interval 1.60–2.33) in the year prior to alemtuzumab initiation to 0.28 (0.17–0.37) after 1 year of treatment (87% reduction), and to 0.22 (0.13–0.35) after the second year. Over the entire follow-up period, ARR was 0.24 (0.18–0.30). At year 1, 75% of patients showed no signs of magnetic resonance imaging (MRI) activity and 70% at year 5. One percent of patients experienced 6-month CDW at year 1, 2.6% at year 2, 7.4% at year 3, and no patients over years 4 and 5. A total of 7.7% of patients achieved 6-month CDI in year 1, 3.6% in year 2, and maintained it at years 3 and 4. Most patients achieved annual NEDA-3: year 1, 72%; year 2, 79%; year 3, 80%; year 4, 89%; year 5, 75%. Infusion-related reactions were observed in 95% of patients and infections in 74%. Thyroid disorders occurred in 30% of patients, and only three patients developed immune thrombocytopenia. No cases of progressive multifocal leukoencephalopathy were reported.
Conclusions
This study shows that alemtuzumab reduced the relapse rate and disability worsening in real-world clinical practice, with many patients achieving and sustaining NEDA-3 over time. The safety profile of alemtuzumab was consistent with previous findings, and no new or unexpected safety signals were observed. As this was an observational and retrospective study, the main limitation of not having all variables comprehensively available for all patients should be considered when interpreting results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In a cohort of patients with relapsing-remitting multiple sclerosis diagnosed for a mean of 7 years, and most treatment-experienced, two courses of alemtuzumab may reduce disease activity and stabilize disability. |
The absence of new and unexpected safety signals during treatment with alemtuzumab adds to the current manageable safety profile of the drug. |
These data are derived from a patient population with different follow-up times and a limited number of participants from year 4 and beyond. Therefore, further prospective studies in cohorts with prolonged and similar follow-up are necessary to validate these results and confirm the long-term benefits of alemtuzumab. |
1 Introduction
The current landscape of new disease-modifying therapies (DMTs) has improved the course of patients with multiple sclerosis (MS) by reducing relapses and disability progression and improving health-related quality of life. These advances enable clinicians to personalize treatments better and maximize benefit for each patient.
Alemtuzumab (Lemtrada®, Sanofi) is a humanized monoclonal antibody, approved for relapsing-remitting MS (RRMS), administered as an intravenous infusion of two courses of 12 mg/day on 5 consecutive days at baseline and then 3 consecutive days 12 months later. Alemtuzumab selectively binds to CD52, mainly in T (CD3+) and B (CD19+) cells, depleting the lymphocyte population most likely through antibody-dependent cell-mediated cytolysis followed by a distinct pattern of repopulation. This alteration in the distribution of lymphocyte subsets is apparently responsible for the long-term success of the drug [1]. While suppression of the central nervous system inflammation through lymphocyte depletion may underlie the reduction in relapses and radiological activity, other clinical effects may be related to alemtuzumab’s ability to modify the immune system during the repopulation process [1].
The efficacy of alemtuzumab in reducing the risk of relapses, disability worsening, and brain volume loss has been demonstrated in clinical trials in both treatment-naive (CAMMS223, CARE-MS I) [2, 3] and previously treated patients (CARE-MS II) [4], and has been confirmed by long-term data, including no evidence of disease activity (NEDA-3), in extended 5-, 9-, and 12-year follow-up [5,6,7,8]. Observational studies, although limited, have supported the safety and effectiveness of alemtuzumab in the main clinical and radiological outcomes used in clinical practice [9,10,11,12,13,14,15,16,17]. Despite methodological differences, they continue to provide valuable information for clinicians managing MS patients currently treated with the drug. In this real-world study, we aimed to assess the benefit–risk profile associated with alemtuzumab in an observational cohort of relapsing MS patients who started treatment between 2014 and 2020. During the study period, the EU label for alemtuzumab was revised [18], and therefore, this cohort of MS patients may reflect the heterogeneity with the current indication.
2 Methods
2.1 Study Design and Patient Population
LEMCAM was a retrospective, multicenter, observational study conducted in five hospitals in Madrid (Spain), adhering to the Declaration of Helsinki and national regulations. Inclusion criteria were age ≥ 18 years, a diagnosis of RRMS, and having received at least one dose of alemtuzumab between 2014 and 2020 as per local labeling and clinical practice. Patients who had participated in clinical trials on alemtuzumab were excluded. The study received approval from the ethics committee of “Hospital Ramón y Cajal” (Madrid, Spain), and all patients gave their written informed consent.
2.2 Assessments
Data retrieved from medical records included demographics, medical history of MS, protocol-defined relapses (new or worsening neurological symptoms attributable to MS lasting at least 24 h, without pyrexia, and after at least 30 days of stability, with a change in neurological examination [19]), Expanded Disability Status Scale (EDSS), magnetic resonance imaging (MRI) activity (new T1 gadolinium [Gd]-enhancing and new T2 lesions), and oligoclonal bands. Clinical outcomes analyzed included the following: annualized relapse rate (ARR), 6-month confirmed disability worsening (CDW; defined as a ≥ 1.0-point increase in EDSS score for patients with a baseline EDSS score ≥ 1 [≥ 1.5 if baseline EDSS = 0], confirmed over 6 months); 6-month confirmed disability improvement (CDI; defined as a ≥ 1-point EDSS score decrease in patients with a baseline score ≥ 2.0, confirmed over 6 months), and NEDA-3. Additional courses and reasons for retreatment were collected.
Safety was evaluated by reviewing the adverse events (AEs) that occurred during the infusion (infusion-related reactions [IRRs]) and during total follow-up (including infections and secondary autoimmunity). Pregnancies occurring during the study were also registered.
2.3 Statistical Considerations
Data are presented using central tendency and dispersion (mean, standard deviation, median and interquartile range [IQR] values) for quantitative variables and counts (percentages) for categorical variables.
The follow-up rate was calculated using patients with date of end of follow-up available.
ARR was calculated using the person-years method, which divides the total number of relapses among all patients divided by the total follow-up period of the study cohort and the 95% confidence interval (CI). This method is used when the follow-up period varies between participants, and takes into account the length of follow-up in each patient. ARR was calculated using patients with complete data on number of relapses during follow-up and follow-up time.
The analysis of 6-month CDW and 6-month CDI was performed on patients with an available baseline EDSS score (the last EDSS score recorded before alemtuzumab initiation) and available EDSS scores during the follow-up time analyzed separated by the confirmation period of 6 months. NEDA-3, defined as the absence of relapses, 6-month CDW, and both new Gd-enhancing or new/enlarging T2 lesions, was evaluated annually and cumulatively. Safety data are reported as the percentage of patients with at least one event and the number of cases per event.
Analyses were based on available data (without missing considered), and values of p < 0.05 were considered significant. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 22 (SPSS Inc., Chicago, USA).
3 Results
3.1 Patient Characteristics
A total of 115 patients were included and evaluable for the analysis (71% women). The mean age at diagnosis was 27.5 ± 7.8 years, and the mean disease duration at alemtuzumab initiation was 7.5 ± 6.4 years (Table 1). Patients had, on average, 1.1 ± 0.9 relapses in the previous year and a mean baseline EDSS score of 3.3 ± 1.6.
Ninety-seven patients (84.3%) had been previously treated, with a mean of 2.9 ± 1.6 DMTs, and 18 (15.6%) were treatment-naive. The last MS treatment before alemtuzumab was fingolimod in 34 patients (35%) and natalizumab in 30 (30.9%) (Table 2). Patients were switched to alemtuzumab mainly because of a lack of effectiveness (70.8%), followed by positivity for anti-John Cunningham virus antibodies and increased risk of progressive multifocal leukoencephalopathy (PML) (22.9%).
One-hundred and four patients (90.4%) received the two courses of treatment. Twenty-five patients received the second cycle delayed (for a mean of 84 days) due to infection (n = 9) or lymphopenia (n = 9). Eleven patients needed a third course (seven due to relapses, one due to activity on MRI, and two due to both relapses and MRI activity). For one patient, the reason was not given. No further courses were administered. Eight patients (7%) discontinued alemtuzumab due to a lack of effectiveness (n = 4), safety (n = 1), and pregnancy (n = 1). In two cases where a third cycle of alemtuzumab was indicated because of relapses and radiological activity, ocrelizumab was administered according to patients’ preference.
Median follow-time was 2.7 years (IQR 1.52). Of the 94 patients with date of end of follow-up available, six patients (6.4%) completed up to 1 year of follow-up, 18 (19.15%) up to 2 years, 31 (33%) up to 3 years, 23 (24.5%) up to 4 years, 13 (13.8%) up to 5 years, and three (3.2%) more than 5 years.
3.2 Effectiveness
The ARR decreased from 1.9 (95% CI 1.60–2.33) in the year prior to alemtuzumab initiation to 0.28 (0.17–0.37) in the first year after treatment (87.3% reduction), 0.22 (0.13–0.35) in the second year after treatment (88.4% reduction), and 0.24 (0.18–0.30) when considering the whole follow-up after treatment (Fig. 1). During the 5 years of follow-up, the percentage of patients free from relapses was 73.9%. By year, the percentage of patients free from relapses was 86.8% at year 1 (99/114), 87.2% at year 2 (89/102), 89.5% at year 3 (68/76), 86.8% at year 4 (33/38), and 68.7% at year 5 (11/16). The percentage of patients free from radiological activity (no new T1 Gd-enhancing and no new T2 lesions) was 75% at year 1 (78/104), 90.6% at year 2 (77/85), 88% at year 3 (44/50), 95.4% at year 4 (21/22), and 70% at year 5 (7/10).
ARR prior to start of alemtuzumab and different periods after treatment. The numbers above the bars show the mean ARR and the 95% CI. The increase in ARR observed at year 5 might be explained by the small number of patients with 5 years of follow-up and therefore the shorter total follow-up period of these patients. The ARR was calculated using the person-years method, which applies the procedure of weighted summing adjusted for each participant's follow-up period. ARR annualized relapse rate, CI confidence interval
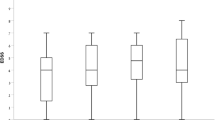
Mean EDSS score remained stable at all examined timepoints (Fig. 2). One of 99 patients experienced 6-month CDW at year 1 (1%), two of 78 patients at year 2 (2.6%), and four of 54 patients at year 3 (7.4%). Over years 4 and 5, no patients of 23 and ten, respectively, experienced 6-month CDW. In year 1, 7.7% of patients achieved 6-month CDI, 3.6% in year 2 and 2.5% in years 3 and 4. No patients achieved 6-month CDI at year 5 (Fig. 3).
Six-month CDI. N is the number of patients with available baseline EDSS score (the latest recorded before alemtuzumab initiation) and available EDSS scores during the follow-up time analyzed separated by the 6-month confirmation period; n is the number of patients who achieved CDI. CDI confirmed disability improvement, EDSS Expanded Disability Status Scale
The percentage of patients achieving annual NEDA-3 was consistent in each year of the study for 5 years (Fig. 4). Of the eight patients with data in all 5 years, three (37.5%) maintained NEDA-3 after 5 years of the first alemtuzumab infusion.
Annual and cumulative NEDA-3. NEDA-3 is defined as the absence of relapses, 6-month CDW, and both new Gd-enhancing or new/enlarging T2 lesions. N is the total number of patients with all data required available; n is the number of patients reaching the NEDA-3 status. CDW confirmed disability worsening, Gd gadolinium, NEDA no evidence of disease activity
3.3 Safety
A total of 109 patients (95%) experienced 219 IRRs. Given the total population, 83.5% of patients experienced rash, 79.1% urticaria, 65.2% headache, and 33% pyrexia. In addition, 85 patients (74%) developed 197 infections, mainly respiratory (79 cases, 40%), urinary (30 cases, 15%), and herpetic infections (23 cases, 12%), and gastroenteritis (22 cases, 11.1%). Secondary autoimmune events comprised thyroid abnormalities in 34 patients (30%) (hyperthyroidism in 17 patients [15%] and hypothyroidism in 17 patients [15%]), and, less frequently, immune thrombocytopenia (ITP) in three patients (3%). Seventy-six patients (66%) suffered 123 laboratory abnormalities, mainly lymphopenia (82 cases, 67%). Six pregnancies were documented with no safety issues, and no deaths occurred. No cases of PML were reported.
4 Discussion
The LEMCAM study shows the experience with alemtuzumab in a real-life cohort of 115 patients treated between 2014 and 2020 and followed a median of 2.8 years in five hospitals in Spain. Results concluded that in patients diagnosed with RRMS for a mean of 7 years, most of them treatment-experienced, two courses of alemtuzumab led to clinical remission and EDSS stabilization, with no unexpected safety issues. Patients were more likely to experience CDI than CDW over time.
Despite being a clinically active population, with a mean of one relapse in the previous 12 months, patients in LEMCAM achieved clinical disease control with a significant reduction (87%) in the ARR from baseline in year 1 after treatment with alemtuzumab, which remained low from 2 to 4 years, as well as favorable radiological outcomes. However, caution should be exercised when interpreting data from year 4 and beyond due to the limited number of patients.
These findings are consistent with other observational studies supporting the effectiveness of alemtuzumab on clinical activity in patients with different treatment histories, regardless of the number of prior relapses and level of disability. If we focus on two of the larger cohorts, the TREAT-MS study showed similar longitudinal reductions in ARR and sustained EDSS from baseline status [16] in mainly pretreated patients, with more relapses and lower EDSS scores than patients in LEMCAM before treatment with alemtuzumab [20]. Russo et al. [13] found a decrease in disease activity comparable to LEMCAM in patients with similar EDSS scores and lower baseline ARR. The cumulative improvement in disability after 2 years of alemtuzumab was, however, lower in LEMCAM.
Two recently published studies, one of which was conducted in Spain, also showed significant reductions in ARR in cohorts that differ notably in terms of patient age, disease duration, and disability [10, 14]. We observed very low rates of disability worsening during the first 2 years of starting treatment with alemtuzumab, and no patient experienced 6-month confirmed disability progression over years 4–5. The probability of CDI was higher than the probability of CDW. The rates of CDI observed in our cohort are lower than those reported in the CARE-MS I and CARE-MS II clinical trials and their respective 5-year extensions [2, 4,5,6], which ranged from 22 to 43%. This is probably because our patients had higher disease activity and disability, were older, and had received more previous treatments before alemtuzumab, the latter two being associated with worse outcomes [21]. The differences we also observed in the proportion of patients achieving CDI compared to other real-world studies (from 11.8 to 48.8%) [9, 10, 12, 14] may be the result of the diverse range of alemtuzumab-treated patients in clinical practice. When interpreting our CDI results, it is important to consider the stability of the EDSS scores in this cohort without significant improvements, as the CDI is a measure that captures sustained and clinically meaningful changes.
The proportion of patients who reached NEDA-3 at 1, 2, and 3 years were 72%, 79%, and 80%, respectively, and 37% maintained NEDA-3 status through the 5-years of follow-up. Although differences in methods and populations make comparison challenging, these results mirror those obtained in the extension of the CAMMS223 trial (as high as 63–78% per year) [7] and CARE-MS I (62% at 3-year follow-up) in naive patients [6], the extension of CARE-MS II after mainly two DMTs (53% at 3-year follow-up) [5], and the observational cohorts of Russo et al. (58.9% at 2-year follow-up) [13], Prosperini et al. (45% at 3-year follow-up) [12], and di Ioia et al. (67% at 3-year follow-up) [9] in patients exposed to a mean of three and four prior treatments. In LEMCAM, treatment with alemtuzumab led to an overall high NEDA-3 rate irrespective of previous DMT, including in treatment-naive patients (16%).
Alemtuzumab has a special dosing regimen, which may partially explain the low discontinuation rate we found. In our study, only 3% of patients discontinued alemtuzumab due to a lack of effectiveness and fewer than 1% for safety reasons. Safety findings were consistent with those reported in clinical trials, despite differences in age, disease duration, baseline EDSS, and previous treatments, and no new safety concerns were identified in our practice setting. As expected, the most frequent AEs were IRRs, generally easily managed, but the incidence was higher in LEMCAM (95%) than in the recent real-world cohorts of Brecl Jakob et al. (59%) [22], Bose et al. (80%) [21], and di Ioia et al. (78%) [9]. Infections were also more frequently reported in LEMCAM (74%) than in these cohorts (ranging from 2.8% to 27.5%) [9, 12, 21, 22]. However, we found a lower incidence of thyroid autoimmune disorders than in those studies and a similar incidence of ITP [9, 21, 22]. Given that safety was a major concern for the investigators, AEs were thoroughly collected regardless of the possible relation to the drug. The main limitations are the retrospective nature of the study, as the data were derived from medical records not intended for research purposes and therefore some information may be missing, and the lack of a comparator group for reference. We acknowledge the likely heterogeneity in clinical management due to the multicenter nature of the study and that a systematic follow-up performed with standardized timings would have strengthened the study results. Also, the different follow-up periods between the study participants and the small number of patients from year 4 and beyond limit the ability to draw conclusions about the long-term benefits of alemtuzumab.
5 Conclusions
The LEMCAM study demonstrates that alemtuzumab may lead to clinical and radiological remission in relapsing MS with different treatment backgrounds. It also shows that alemtuzumab may prevent disability progression and is effective in achieving the NEDA-3 status. The safety profile of alemtuzumab in this real-world population aligns with that seen in clinical trials and other observational cohorts, and no new and unexpected safety findings were observed. These results need to be confirmed in other groups of patients with longer follow-up, but they are considered important in supporting the effectiveness and particularly the safety of alemtuzumab, as its use has been limited due to safety concerns.
References
Freedman MS, Kaplan JM, Markovic-Plese S. Insights into the mechanisms of the therapeutic efficacy of alemtuzumab in multiple sclerosis. J Clin Cell Immunol. 2013;4(4):1000152.
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. The Lancet. 2012. https://doi.org/10.1016/S0140-6736(12)61769-3.
Investigators CT, Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008. https://doi.org/10.1056/NEJMoa0802670.
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. The Lancet. 2012. https://doi.org/10.1016/S0140-6736(12)61768-1.
Coles AJ, Cohen JA, Fox EJ, Giovannoni G, Hartung HP, Havrdova E, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology. 2017. https://doi.org/10.1212/WNL.0000000000004354.
Havrdova E, Arnold DL, Cohen JA, Hartung HP, Fox EJ, Giovannoni G, et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology. 2017. https://doi.org/10.1212/WNL.0000000000004313.
Steingo B, Al Malik Y, Bass AD, Berkovich R, Carraro M, Fernández Ó, et al. Long-term efficacy and safety of alemtuzumab in patients with RRMS: 12-year follow-up of CAMMS223. J Neurol. 2020. https://doi.org/10.1007/s00415-020-09983-1.
Ziemssen T, Bass AD, Berkovich R, Comi G, Eichau S, Hobart J, et al. Efficacy and safety of alemtuzumab through 9 years of follow-up in patients with highly active disease: post hoc analysis of CARE-MS I and II patients in the TOPAZ extension study. CNS Drugs. 2020. https://doi.org/10.1007/s40263-020-00749-x.
di Ioia M, Di Stefano V, Farina D, Di Tommaso V, Travaglini D, Pietrolongo E, et al. Alemtuzumab treatment of multiple sclerosis in real-world clinical practice: a report from a single Italian center. Mult Scler Relat Disord. 2020. https://doi.org/10.1016/j.msard.2019.101504.
Eichau S, Lopez Ruiz R, Ruiz de Arcos M, Ruiz-Pena JL, Navarro G, Calleja MA, et al. Results of treatment with alemtuzumab in a Spanish cohort of patients with multiple sclerosis in the real world: The RealMS study. Front Neurol. 2023. https://doi.org/10.3389/fneur.2023.1112193.
Frau J, Coghe G, Lorefice L, Fenu G, Musu L, Cocco E. Efficacy and safety of alemtuzumab in a real-life cohort of patients with multiple sclerosis. J Neurol. 2019. https://doi.org/10.1007/s00415-019-09272-6.
Prosperini L, Annovazzi P, Boffa L, Buscarinu MC, Gallo A, Matta M, et al. No evidence of disease activity (NEDA-3) and disability improvement after alemtuzumab treatment for multiple sclerosis: a 36-month real-world study. J Neurol. 2018. https://doi.org/10.1007/s00415-018-9070-x.
Russo CV, Sacca F, Frau J, Annovazzi P, Signoriello E, Bonavita S, et al. A real-world study of alemtuzumab in a cohort of Italian patients. Eur J Neurol. 2022. https://doi.org/10.1111/ene.15121.
Sandgren S, Novakova L, Nordin A, Axelsson M, Malmestrom C, Zetterberg H, et al. A five-year observational prospective mono-center study of the efficacy of alemtuzumab in a real-world cohort of patients with multiple sclerosis. Front Neurol. 2023. https://doi.org/10.3389/fneur.2023.1265354.
Theodorsdottir A, Debrabant B, Magyari M, Kant M, Rasmussen PV, Malmberg CF, et al. Alemtuzumab treatment in Denmark: a national study based on the Danish Multiple Sclerosis Registry. Mult Scler. 2021. https://doi.org/10.1177/13524585211003291.
Ziemssen T, Hoffmann F, Richter S, Engelmann U, Quint L, Group T-MS. Real-world effectiveness of alemtuzumab in RRMS patients in Germany: Interim results of the TREAT-MS study after completion of recruitment. In: 2021; 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis 2021. p. October 13-5, 2021.
Ziemssen T, Thomas K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord. 2017. https://doi.org/10.1177/1756285617722706.
Last updated 2019 [cited; Available from: https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar-product-information_en.pdf.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2010. https://doi.org/10.1002/ana.22366.
Ziemssen T, Hoffmann F, Richter S, Engelmann U, White R. Alemtuzumab in a large real-life cohort: interim baseline data of the TREAT-MS study. Front Neurol. 2021. https://doi.org/10.3389/fneur.2021.620758.
Bose G, Rush C, Atkins HL, Freedman MS. A real-world single-centre analysis of alemtuzumab and cladribine for multiple sclerosis. Mult Scler Related Disord. 2021. https://doi.org/10.1016/j.msard.2021.102945.
Brecl Jakob G, Barun B, Gomezelj S, Gabelic T, Sega Jazbec S, Adamec I, et al. Effectiveness and safety of alemtuzumab in the treatment of active relapsing-remitting multiple sclerosis: a multicenter, observational study. Neurol Sci. 2021. https://doi.org/10.1007/s10072-021-05145-x.
Acknowledgments
The authors are very grateful to all the patients for participating in the LEMCAM study. The authors take full responsibility for the content of the article and thank Isabel Caballero from Evidenze Health España, S.L.U for support with the medical writing, literature searching, and journal styling, funded by Sanofi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study, manuscript preparation, and open access was funded by Sanofi.
Competing Interests
EM declares receipt of consulting and advisory board fees from Sanofi. He reports receiving research grants, travel support, or honoraria for speaking engagements from Biogen, Sanofi, Merck, Novartis, Almirall, Roche, BMS, and Janssen outside the submitted work. CA declares receipt of grants, travel support, or honoraria for speaking engagements from Biogen, Janssen, Merck, Novartis, Roche, and Sanofi. JSM declares receipt of travel and educational support or honoraria for speaking engagements from Almirall, Biogen, Janssen, Merck, Novartis, Roche, Sanofi, and Teva. SSdM declares receipt of travel support, honoraria for speaking, and consulting fees from Sanofi. JPC declares receipt of consulting fees, support for travel, fees honoraria for participation on data monitoring boards, speaking honoraria, and expert testimony from Novartis, Biogen, Merck, Sanofi, and Roche. JLCG has received honoraria for speaking engagements or consulting services from Biogen, Bayer, Bial, and Sanofi. SMM is an employee of Sanofi. JMGD has received fees as speaker or advisor and travel or research grants from Almirall, Biogen, Roche, Sanofi, Janssen, BMS, Merck, and Novartis. LCFF, VML, ALF, RB, MLMG, CDP, ALR, and FRJ declare that they have no competing interests.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki and national regulations. Approval was granted by the Ethics Committee of Hospital Ramón y Cajal (Madrid, Spain).
Consent to Participate
All patients provided written informed consent for the collection of data from their medical records.
Consent for Publication
Not applicable.
Availability of Data
Data supporting the results reported in the article will be made available from the corresponding author upon request.
Code Availability
Not applicable.
Author Contributions
LCFF, VML, SMM, and JMGD made substantial contributions to the conception and design of the work, interpretation of data, and drafting the manuscript. All authors contributed to the acquisition of data and revised the manuscript critically for important intellectual content. All authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Costa-Frossard França, L., Meca Lallana, V., Labiano-Fontcuberta, A. et al. Real-World Retrospective Analysis of Alemtuzumab Outcomes in Relapsing-Remitting Multiple Sclerosis: The LEMCAM Study. CNS Drugs 38, 231–238 (2024). https://doi.org/10.1007/s40263-024-01066-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-024-01066-3