Abstract
Stroke remains one of the leading causes of mortality and long-term and permanent disability worldwide despite technological innovations and developments in pharmacotherapy. In the last few decades, the growing data have evidenced the role of the circadian system in brain vulnerability to damage, the development and evolution of stroke, and short-term and long-term recovery. On the other hand, the stroke itself can affect the circadian system via direct injury of specific brain structures involved in circadian regulation (i.e., hypothalamus, retinohypothalamic tracts, etc.) and impairment of endogenous regulatory mechanisms, metabolic derangement, and a neurogenic inflammatory response in acute stroke. Moreover, the disruption of circadian rhythms can occur or exacerbate as a result of exogenous factors related to hospitalization itself, the conditions in the intensive care unit and the ward (light, noise, etc.), medication (sedatives and hypnotics), and loss of external factors entraining the circadian rhythms. In the acute phase of stroke, patients demonstrate abnormal circadian variations in circadian biomarkers (melatonin, cortisol), core body temperature, and rest–activity patterns. The approaches aimed at the restoration of disrupted circadian patterns include pharmacological (melatonin supplementation) and non-medication (bright light therapy, shifting feeding schedules, etc.) interventions; however, their effects on short- and long-term recovery after stroke are not well understood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Increasing evidence shows that the circadian system is implicated in the regulation of processes mediating vulnerability to stroke, as well as stroke evolution and post-stroke recovery. |
The interaction between the circadian system and brain damage is bidirectional, and post-stroke circadian rhythm disruption is commonly found via assessment of the circadian variation in the main circadian biomarkers and parameters. The impact of these changes on post-stroke recovery and long-term prognosis is not well established and needs further investigation. |
The approaches to manage circadian disruption include medication (melatonin) and non-medication (bright light therapy and resetting molecular clocks by other external signals, such as shifting feeding schedule and physical activity). The evidence on their applicability and efficiency in patients with stroke is limited. |
Introduction
In 2022, the American Heart Association (AHA) updated and enhanced the approach to assessing cardiovascular health “Life’s Essential 8” [1]. An important update is the inclusion of sleep health as a new crucial component in optimizing and preserving cardiovascular health, because sleep metrics provide an independent additional predictive value for cardiovascular events above the original seven metrics of cardiovascular health (diet, physical activity, nicotine exposure, body mass index, blood lipids, blood glucose, and blood pressure). The AHA, mainly focusing on sleep duration, emphasizes the need for the investigation of other sleep metrics in relation to cardiovascular health. Sleep health extends far beyond the recommended duration of sleep [2, 3], as it is a multidimensional construct with multiple interacting components (duration, timing, regularity, efficiency, satisfaction, and impact on daytime alertness) [4] and it should also be considered as a part of the circadian sleep–wake cycle. Increasing evidence highlights the important role of the circadian system in cardiovascular functioning. Based on the 2016 report of the Global Burden of Disease 2016 Lifetime Risk of Stroke Collaborators, the global lifetime risk of stroke for both men and women aged 25 years or older has been increasing in the last 3 decades and is estimated at 25% [5].
This review will focus on the role of circadian factors in stroke, which remains one of the leading causes of mortality and long-term disability worldwide [6, 7]. It is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Circadian Pattern of Stroke Occurrence
Circadian Timing in Different Stroke Types
Like other major cardiovascular events, the incidence of stroke demonstrates an uneven circadian pattern with two peaks, a major one in the morning, between 06:00 and 12:00 [8,9,10,11], and a lower one in the evening [12]. A meta-analysis of 31 publications assessing the circadian timing of 11,816 patients showed such a double-peak distribution in both ischemic and hemorrhagic strokes, although it is more clear in ischemic stroke, and it is independent of age, gender, vascular risk factors (such as hypertension, diabetes mellitus, hyperlipidemia, smoking habits), prior cerebrovascular or cardiovascular events, and previous treatment with antiplatelet or anticoagulant drugs. In ischemic stroke, the morning peak is typical for all TOAST stroke subtypes, but is most pronounced in the large artery atherosclerosis subtype [10]. However, the National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Trial did not find clear circadian variation in the onset of ischemic stroke which might be due to the selection bias as patients admitted to the hospital later than 3 h after symptoms onset were not included in this study [13].
Stroke Occurrence Timing and Prognosis
Although experimental studies confirm the association of both occurrence and brain lesion volume with the time of stroke onset [14], the clinical data on the association between the stroke occurrence time and the prognosis is limited. A retrospective analysis of 3689 patients demonstrated that night-onset stroke survivors had a worse 30-day prognosis, compared to the patients with day-onset strokes. This association was present only in ischemic strokes, and among those, only in the cardioembolic subtype. The difference in the prognosis was explained by the limited therapeutic window for thrombolytic therapy [15]. Similar conclusions were made on the basis of the results of the prospective NINDS rt-PA Stroke Trial [13]: patients with the stroke onset between 00:00 and 06:00 were less likely to get treatment within 90 min. In a recently published large Korean study (n = 17,461), night-onset strokes were more severe and were associated with higher rates of early neurological deterioration and a lower likelihood of a favorable functional outcome compared to day-onset strokes independent of other covariates and revascularization therapy. The association of night-onset (versus day-onset) strokes with early neurological deterioration was significant and most pronounced in the large artery atherosclerosis subtype compared to other stroke subtypes [16]. On the basis of the data from the Japanese Takashima Stroke Registry, morning-onset strokes were associated with a significantly higher risk of a 28-day lethal outcome compared to afternoon-onset strokes independently of age, gender, and stroke severity [17].
The explanations for the diurnal pattern of stroke occurrence include circadian and/or postural changes in some pathophysiological factors observed at awakening and resumption of physical and mental activities such as increased platelet aggregation, decreased thrombolysis, proinflammatory factors, surges of blood pressure (BP) and heart rate (HR), and higher catecholamine levels, reflecting a peak in circadian sympathetic activity along with the increased activity of the renin–angiotensin–aldosterone system [18, 19].
Circadian Misalignment in Stroke: Cause or Consequence?
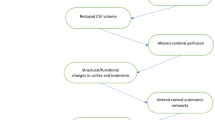
Both experimental and clinical studies provide evidence of the impact of circadian misalignment on stroke development (Fig. 1), including an increase in the brain lesion volume, demonstrated worse functional dysfunction, and higher mortality which is likely due to the imbalance in the expression of pro- and anti-inflammatory cytokine genes in the brain [20,21,22]. However, some authors suggest that single light-induced circadian disruption is not potent enough to cause increased cerebral vulnerability to ischemia, and other concomitant factors or diseases are required [23]. Cantone et al. demonstrated that exogenous air pollution could lead to the reprogramming of the molecular circadian system (clock genes), which is associated with the severity of subsequent stroke (assessed by the National Institutes of Health Stroke Scale (NIHSS) score) and 3-month disability (evaluated by the modified Rankin Scale, mRS) [24].
The circadian misalignment appears to be a potential risk factor for cardiovascular events including stroke. Even short-term (12-h) circadian misalignment simulating a shift-work inversion of the behavioral cycle results in the increase in 24-h BP, reduced BP dipping, enhanced inflammatory markers (such as C-reactive protein, tumor necrosis factor alpha, resistin, and interleukin-6), and caused autonomic imbalance with diminished parasympathetic tone [25]. In a large Japanese cohort (n = 78,115), a self-reported irregular daily routine was significantly associated with an increased risk of incident cardiovascular diseases including stroke in women but not in men [26]. A meta-analysis of five studies by Li et al. did not find an increased risk for the development of ischemic stroke (non-significant combined RR, 1.03; 95% CI 0.99–1.07) [27]. However, the association between shift work and incident stroke might be modified by the genetic variants, i.e., melatonin receptor type 1B (MTNR1B) rs10830963 polymorphism (G-allele displaying a protective role while C-allele being unfavorable) or other factors [28].
Abnormal patterns in the circadian variation of BP (non-dipping or extreme dipping [18, 19]) and HR [29] observed in diseases (and sometimes considered a sign of circadian misalignment) represent predictive factors for stroke, independently of awake and sleep systolic BP [30, 31]. On the other hand, the available evidence indicates that the abnormal circadian BP patterns in acute stroke can develop secondary to stroke resulting from the stroke-related autonomic imbalance. Thus, patients in the acute phase of stroke demonstrate diminished BP variability throughout 24 h and decreased day–night BP difference. These abnormalities correlate with the changes in the circadian variability in other neurohumoral factors, in particular, the levels of endothelin-1 and brain natriuretic peptide (BNP) [32,33,34]. Patients with acute stroke also demonstrate increased catecholamine levels. The loss of the circadian BP profile and non-dipping patterns are also common (up to 90% of patients with stroke) [35,36,37,38]. Some authors report the association between the abnormal circadian BP pattern and the specific location of the brain lesion [33, 36] and other characteristics (e.g., the presence of cortical microbleeds [39], asymptomatic lacunar infarcts [40], and other signs of small vessel disease [41], brain atrophy [42]), although the data are controversial. Some results suggest that post-stroke survivors with abnormal circadian BP profiles (non-dippers, reverse dippers) are more likely to have a poor outcome [38], although some reports are conflicting [43]. Makikallio et al. found elevated levels of cortisol and natriuretic peptides in half of patients with stroke who died during the follow-up versus stroke survivors. Cortisol levels were associated with atrial natriuretic peptide (N-ANP) and N-BNP levels, catecholamine levels (r = 0.55–0.94, p < 0.01 for all), and measures of neurologic deficit (r = 0.36–0.44, p < 0.05) on the 2nd and 7th days. High acute-phase cortisol levels assessed either in the morning (RR = 5.4, p < 0.05) or in the evening (RR = 5.8, p < 0.05) predicted long-term mortality after stroke analysis [44].
An imbalance in prothrombotic (plasminogen activator inhibitor 1, PAI-1) [45] and antithrombotic (tissue factor pathway inhibitor) [46] factors, which show clear antiphasic 24-h rhythms independent of the sleep–wake cycle, may play a contributing role to circadian variation in stroke occurrence [47].
The potential impact of sleep-related factors and disorders should be also considered. The autonomic instability and abrupt surges of sympathetic activity are typical in REM sleep periods that are the longest in the early morning hours before awakening [48, 49]. Sleep disorders per se can be a contributing factor, and among them, sleep apnea is the most significant. Untreated severe OSA doubles the risk of stroke. According to expert consensus, the role of insomnia, periodic limb movements, and circadian misalignment is disputable and lacks solid evidence [50].
On the other hand, the stroke itself can result in the disruption of circadian rhythms. Direct cerebral injury, compression of hypothalamic structures, and impairment of endogenous regulatory mechanisms, as well as changes in cerebral blood flow, metabolic derangement, and a neurogenic inflammatory response are the main factors underlying circadian disruption in acute stroke. In addition, the disruption of the diurnal sleep/wake pattern due to exogenous factors, such as hospitalization itself, the conditions in the intensive care unit, light conditions, loss of external zeitgebers of circadian rhythm, and the use of sedative or hypnotic drugs can affect circadian patterns [51, 52].
Circadian System and Stroke
Circadian System Structure and Regulation
In mammals, the circadian system comprises a master pacemaker located in the suprachiasmatic nucleus (SCN), which coordinates and synchronizes cellular clocks in various tissues and organs.
The circadian system is self-regulated and at the cellular level functions via interaction between circadian genes (the core genes include CLOCK, BMAL1, two cryptochrome genes CRY1 and CRY2, three period genes PER1, PER2, PER3) and their protein products. The oscillation cycle of the transcription–translation feedback loop is about 24 h entrained by the light–dark cycle. The positive arm of the feedback loop involves the protein products of circadian genes CLOCK (or NPAS2 in brain tissue) and BMAL1. They form a heterodimer in the cytoplasm which is translocated to the cell nucleus where it binds to the E-box promotion region of the circadian genes Period (PER) and Cryptochrome (CRY) driving their translation. In the negative arm of the loop, the Per and Cry proteins form a heterodimer in the cytoplasm translocating to the nucleus, where it binds to the complex Clock–Bmal1 and thereby suppresses the transcription–translation cascade [53, 54]. Another pair of a regulatory loop includes circadian genes encoding nuclear receptors Rev-erbα and RAR-related orphan receptors (RORα) binding the promoter of the BMAL1 gene.
Routinely, the external signals reset molecular clocks, and the main synchronizer is light (photic signals); however, the circadian clocks (in particular, peripheral ones) can be also entrained by neural, humoral, and metabolic (food-induced) signals [55].
Circadian Genes in Stroke
Circadian genes can play a role in stroke incidence, evolution, and recovery via direct modulation of neuronal damage and recovery and their impact on various cardiometabolic factors and processes [47, 56,57,58]. Clock genes modulate microglia activation [59] and neuroinflammation [60, 61], thus regulating the vulnerability and tolerability to brain injury, demonstrating neuroprotective effects [62,63,64,65].
The G-allele of the CLOCK gene was shown to be protective against stroke in the PREDIMED (PREvencion con DIeta MEditerranea) trial cohort; however, it is noteworthy that the association between the CLOCK gene rs4580704 single nucleotide polymorphism (SNP) and stroke risk was found only in the subcohort of patients with type 2 diabetes mellitus [66], but not in subjects without diabetes. Other genetic variants of the CLOCK gene were shown to be involved in the metabolic traits pathways [67], suggesting the potential impact of the CLOCK gene on cardiovascular risk.
Circadian genes are involved in vascular function, tone regulation [68, 69], coagulation factors, and thrombogenicity [70, 71] as shown by experimental and clinical studies. It is noteworthy that different circadian genes have a discrete impact on the circadian variation of these factors. Thus, some authors suggest that Bmal1 and CLOCK are essential for circadian blood pressure oscillations while NPAS2 is important for the precise timing of the rhythmic variability in the presence of CLOCK and can substitute the latter one in the case of CLOCK gene mutation/deficiency [68, 69]. CLOCK and BMAL1 are essential for maintaining a diurnal variation in thrombogenesis, PAI-1 expression, and display opposite effects on time to vessel occlusion [70, 71].
PER2 mutation is associated with impaired endothelial function independent of cardiometabolic risk factors, as well as the alteration in a diurnal change in endothelial-mediated vascular response. On the other hand, the deficiency of PER2 plays a cardioprotective role due to a lower apoptosis rate and lower macrophage infiltration [56, 72, 73]. This can be partly explained by the hypoxia-independent but hypoxia-inducible factor 1-alpha (HIF-1α)-mediated upregulation of vascular growth factors and angiogenesis [74]. One can hypothesize that in various tissues, deficiency or impairment of circadian genes might have different effects playing either protective or deleterious roles depending on the synchronization/desynchronization state between the SCN and peripheral clocks, as well as other factors.
Alternatively, clock genes are tuned via DNA methylation, one of the mechanisms of gene expression regulation [75] that is sensitive to environmental pollution, e.g., particulate matter. In 55 patients with acute stroke, Cantone et al. evaluated the effects of exposure to particulate matter experienced before stroke on clock gene methylation levels, in order to investigate their possible role in modulating patients’ prognosis after the event. They found that clock gene methylation was modified by short-term particulate matter exposure. Moreover, DNA methylation of the studied clock genes (i.e., CRY1, PER1) was associated with the neurological disability assessed by mRS [24]. An increased level of low-grade inflammation and prothrombotic state is suggested as the biological link between exposure to pollutants and deteriorating cardiovascular health and can be mediated by DNA methylation at clock genes.
Assessment of Circadian Rhythm Parameters in Stroke
Challenges in Circadian Rhythm Evaluation in Patients with Stroke
The evaluation of the circadian profile includes various approaches, i.e., subjective (questionnaires, e.g., Horne–Östberg Morningness Eveningness Questionnaire (MEQ) and the Munich Chronotype Questionnaire (MCTQ); sleep logs) and objective ones (laboratory biomarkers such as melatonin, cortisol diurnal patterns; rest–activity periods by actigraphy; core body temperature pattern, etc.). However, in patients with stroke, implementation of these methods can be limited because of neurological deficits (altered consciousness, cognitive decline, amnesia, aphasia, etc.) and other post-stroke changes. Thus, paresis, low activity, as well as micromovements of the limbs can impede the actigraphy results [76], although recent developments suggest various solutions to overcome these problems (i.e., armband devices [76] or ankle fixation of the device [77, 78], the calculation of specific indices, such as the Asymmetry Rate Index [79] for detecting the asymmetry of spontaneous movements between the paretic and non-paretic arm; or the Proportional Integrating Measure [80] which is the ratio between the mean motor activities of both arms [67]). Post-stroke xerostomia prevalent in up to 50–60% of stroke survivors [81] might limit saliva sampling, while post-stroke hyperthermia (a common feature in diencephalic strokes) can affect the circadian profile of core body temperature [82, 83]. Melatonin is a reliable circadian marker not affected generally by the sleep–wake cycle, mood changes, stress factors, etc. Cortisol displays a clear circadian variation, although the influence of some environmental factors can be significant. Moreover, the results may vary depending on stroke characteristics, such as stroke severity, location, the phase after stroke onset (acute, subacute), etc. [84].
Cortisol
The diurnal pattern of cortisol is commonly disturbed and the cortisol levels are elevated in acute stroke compared to reference levels, while they tend to normalize 1 month post-stroke [85, 86]. The elevated cortisol is associated with a worse prognosis, more severe motor dysfunction [87], and post-stroke complications, including post-stroke depression [88]. Murros et al. found that evening cortisol (19:00 at baseline and 1 week after admission to the hospital) better predicted the prognosis and severity of neural deficit than morning cortisol (07:00) [87]. Also, patients with a more severe stroke demonstrate a loss of the circadian pattern [85, 89] or a more disturbed diurnal variation of cortisol, compared to mild strokes [90, 91]. The majority of studies evaluated cortisol once or twice daily [85,86,87,88], with only a minority performing multiple measurements [90, 91] during the day. The latter confirms that in a non-severe stroke, the circadian variations of cortisol are preserved although they demonstrate an advanced circadian rhythm with elevated values at each time point compared to controls [64].
Melatonin
Similarly, the majority of studies vary by time of the collection of samples, the type of biosamples (serum, saliva, urine), the method of melatonin assessment, etc., which limits the direct comparisons of the results. The majority of studies report abnormal melatonin levels and/or patterns in patients with stroke [92,93,94]. Thus, in a cohort of 127 patients with anterior strokes, the urinary excretion of both melatonin and its metabolite 6-sulfatoxymelatonin was significantly reduced compared to healthy individuals [93]. Fiorina et al. showed decreased levels of nocturnal melatonin urinary excretion independent of the size of the cortical lesion which remained low by the 14th day post-stroke [94]. The lesions in the brainstem, lateral and third ventricles, basal ganglion, frontal, frontoparietal, and parietotemporal lobes are associated with the abolished circadian rhythm of melatonin [89]. On the other hand, Beloosesky et al. found a similar total 24-h amount of melatonin in patients with stroke and controls, with the preserved although 4-h delayed melatonin secretion in the first post-stroke days and restoration of the normal pattern by the 10th day after stroke onset [95]. It should be noted that this study included patients with extensive cortical and deep strokes of both hemorrhagic and ischemic types. The authors explain the delayed rhythm of melatonin by potential impairment in light perception at the SCN and consider it as a factor contributing to post-stroke hypothalamic-glandular axis dysregulation. In moderate stroke, not involving directly the regions regulating melatonin pineal production, the circadian rhythm of serum melatonin is sustained [90, 91], demonstrating a clear although flattened periodic pattern [64]. Adamczak-Ratajczak et al. hypothesize that the lower levels of melatonin are due to its increased rapid utilization for the reduction of oxidative damage within the acute phase of cerebral ischemia [91]. Some authors demonstrated that lower levels of melatonin were associated with a worse prognosis (higher 30-day mortality) [96].
The disruption of the circadian pattern of melatonin in patients with stroke can be related to direct damage to SCN or brain areas participating in the regulation of SCN (paraventricular nucleus of the hypothalamus (PVN), and intermediolateral nucleus of the spinal cord (IML), the thalamic intergeniculate leaflet (IGL), raphe nuclei, parietal cortex) [97, 98] and pineal activity or indirect influences [95], or disorder in autonomic regulation [99].
Core Body Temperature
Acute brain damage is associated with the increase in body temperature and a disruption or inversion in the core and brain temperature rhythm [82, 100,101,102,103] (in particular, in lesions involving the frontotemporal area, hypothalamus, and brainstem [104]). In patients with stroke, the rise in body temperature starts 4–6 h after stroke onset and is related to initial stroke severity, usually observed in major strokes and not evident in patients with mild-to-moderate strokes, although some controversial data are reported depending on the time of temperature measurement, stroke severity, etc. [105, 106]. Hyperthermia within the first 24–72 h after stroke onset (but more than 6–8 h after stroke onset) has an unfavorable prognostic impact being a negative predictor of the 3-month outcome [105, 107, 108]. At the same, time low body temperature at admission less than 6 h after stroke onset is associated with more severe stroke (which some authors explain by the possible detrimental effect of lower temperature on clot formation and lysis) and a negative prognosis at discharge [109] and at a long-term follow-up [108, 110]. In a retrospective study [111], the peak body temperature positively correlated with the NIHSS score, female gender, rectal (vs. tympanic) temperature, dysphagia assessed by swallowing test, intubation, high C-reactive protein or signs of infection at admission, and antipyretic treatment within 48 h.
However, only a few studies evaluated circadian variation in body temperature in patients with stroke. Some clinical observations indicate a severe disruption of temperature circadian rhythms [112]. It seems that the circadian rhythm disruption is more prevalent and profound in more severe strokes, in particular those causing the development of disorders of consciousness when both basal temperature and brain temperature rhythms are severely disrupted (or completely absent) [82, 102, 113]. This observation is confirmed by Takekawa et al. in a mixed group of patients with ischemic cardioembolic stroke (n = 11) and cerebral hemorrhage (n = 22). The circadian rhythm was preserved in conscious patients while infradian rhythms of body temperature with a periodicity of approximately 2.5 days (28.6–125.6 h; median 59.7 h) were present in those with disturbed consciousness (39% of patients). They did not find any association between the circadian vs. infradian rhythm and lesion location, but there was a correlation with the mRS. In a larger cohort of patients with stroke mainly with preserved consciousness (n = 50), the same group found a strong correlation between the mRS at a 3-month follow-up and the disruption of the circadian rhythm assessed by both rectal temperature and wrist motor activity (by an accelerometer) independent of the mRS at admission. No association was found between the diurnal rhythm and the brain lesion location.
Rest–Activity Patterns
The majority of studies of actimetry data in patients with stroke are focused on the assessment of physical activity patterns [114,115,116] or sleep parameters [117,118,119,120], and only a limited number of papers consider actigraphy-evaluated diurnal rhythmicity metrics in post-stroke survivors, such as interdaily stability (IS, characterizing the day-to-day stability of activity patterns); intradaily variability (IV, evaluating the fragmentation of activity rhythms); and the amplitude of rest–activity rhythms [121]. Gonçalves et al. showed that patients with cerebrovascular events had more fragmented rhythms and reduced activity, compared to controls [122]. An autopsy study by Sommer et al. demonstrated that more disrupted 24-h rest–activity rhythms were associated with a higher burden of arteriolosclerosis and subcortical infarcts at death in subjects with verified small vessel disease [123]. In the Rotterdam study, Zuurbier et al. demonstrated an association between small vessel disease and 24-h rhythm fragmentation independent of sleep parameters, although lacunar infarcts were not associated with any of the diurnal rhythmicity measures [124]. The association between 24-h rhythm fragmentation and stroke characteristics and prognosis remains unclear.
The fragmentation of the 24-h rhythm is in accord with sleep–wake disturbances often found in stroke survivors. Bakken et al. investigated sleep–wake patterns and found a large variation in sleep during night-time within the first 2 weeks after stroke occurrence with sleep disturbances registered more often in men than in women [118]. Stroke survivors in the chronic post-stroke phase spend more time in bed with the intention of sleeping and actually asleep and significantly more time awake overnight than healthy controls; however, these measures were unrelated to the severity of motor impairment [120]. It was shown that the derivative indices calculated as ratios between 24-h motor activity registered from both affected and unaffected arm better predicted the prognosis in post-stroke survivors [80, 125]. Thus, the Asymmetry Rate Index correlates with the NIHSS [79] and 3-month mRS [125], while the Proportional Integrating Measure predicted the mRS at 3 months [80].
Circadian Gene Expression
Critically ill patients with hemorrhagic stroke demonstrate complete loss of rhythmicity in circadian gene (CRY1, CRY2, PER1, PER2, PER3, RORA, NR1D1, BMAL1, CLOCK, and TIMELESS) expression in peripheral tissues and no correlation with either rest–activity rhythmicity or melatonin rhythm [83]. The severity of encephalopathy and exposure to adrenergic agonist medications appear to be the main factors contributing to these changes [126, 127].
On the other hand, patients with mild-to-moderate stroke seem to have preserved diurnal variation in peripheral clock genes (NR1D1, PER1, PER3, BMAL1), although they demonstrate specific circadian desynchrony in both positive and negative loops of the circadian system, correlating with the diurnal changes in melatonin and cortisol levels [90]. The association of these changes with prognosis and treatment effects is unknown.
Circadian System and Post-Stroke Recovery
The circadian system is involved in the regulation of tissue repair and regeneration after the injury. Glial cell populations play a crucial role in neuronal recovery after brain injury and display both beneficial and detrimental effects on the ischemic lesion zone [128, 129]. Bmal1 regulates the activation of microglia [130] and astrocytes [131]. Clock genes modulate glutamate uptake by regulating the expression and activity of its transporters, thus being involved in excitotoxicity [132] and neuronal cell death regulation [133]. No less important is the involvement of the circadian gene in the regulation of inflammatory response, including the immune function, i.e., trafficking of immune cells, phagocytosis, secretion of cytokines, chemokines, and other factors [61, 72, 74, 132, 134, 135].
Angiogenesis is an important factor for post-stroke recovery, and it is tightly related to the activation and functioning of glial cells [136]. Clock genes (in particular, Cry1/2) are involved in the regulation of different stages of the angiogenesis process [137, 138]. Multiple angiogenic factors are implicated in the post-stroke angiogenesis, and vascular endothelial growth factor (VEGF) is a protein that is a well-known factor regulating and inducing angiogenesis in various pathological conditions and is associated with elevated transdifferentiation of reactive astrocytes into neuroprogenitors and new neurons [139]. Low-level light at night can lead to a reduction in the hippocampal expression of VEGFA and brain-derived neurotrophic factor (BDNF) and increased VEGFR1 [140].
Clock genes Rev-erbα and RORα modulate the expression of antioxidant enzymes and reactive oxygen species production [141] and can play a protective role in the inflammatory response of microglia [142, 143].
The neurotrophic factors involved in the regulation of post-stroke recovery demonstrate clear 24-h rhythmicity, although influenced by other endogenous factors (i.e., levels of sex hormones) [144]. One of these factors, BDNF, can modulate circadian system functioning via the regulation of SCN plasticity [145, 146]. BDNF could act via GABA (γ-aminobutyric acid), NMDA (N-methyl-d-aspartate), or AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) transmitter systems and regulate the light-induced circadian resetting of the SCN pacemaker. Local paracrine interactions between BDNF and TrkB receptors could provide an endogenous signaling pathway for the intercellular regulation of cellular and metabolic activity within the SCN and thus for pacemaker cell synchronization [145, 147].
Circadian System and Treatment Developments in Stroke
Pharmacotherapy Development
Chronotherapy Approaches
Several therapeutical interventions are being discussed in relation to circadian system functioning in stroke. First, chronotherapy approaches [148], in particular for antihypertensive medications, are promoted by some groups of authors, although recent evidence does not provide any benefit of evening vs. morning administration of antihypertensive drugs regarding long-term cardiovascular outcomes [149, 150].
Other treatments (antiplatelet [151], anticoagulants [152], thrombolytic drugs [153, 154], etc.) might have a greater capacity within the chronotherapy approach, although further investigation is needed [47]. Thus, as mentioned above, there is strong evidence confirming circadian variation in the activity of fibrinolytic and prothrombotic factors, in particular, the balance between the tissue plasminogen activator (tPA) and its inhibitor, the tissue plasminogen inhibitor-1 (PAI-1). The latter shows peak concentrations in the morning, which can contribute to a higher risk of atherothrombotic events, as well as to the lower efficiency of fibrinolytic treatment. In a retrospective analysis, diurnal (from 09:00 to 21:00; n = 92) tPA treatment (intravenously administered alteplase 0.9 mg/kg, 10% in bolus, 1 h infusion) was associated with higher rates of middle cerebral artery (MCA) complete recanalization assessed by transcranial duplex compared to the group which was treated during the night period (from 21:00 to 9:00; n = 43): 45.6% vs. 23.2% (p = 0.01). Moreover, those treated during the day demonstrated better 3-month clinical outcomes evaluated by the mRS [153]. However, the NINDS rt-PA Stroke Trial (n = 624) did not find any association between the stroke onset time (and rt-PA treatment given within 3 h of stroke onset) and 3-month outcomes, although there was an association with the occurrence of a symptomatic intracranial hemorrhage within 36 h: patients with stroke onset between 04:01 and 08:00 who received rt-PA had a lower risk of having a symptomatic intracranial hemorrhage [13]. Some authors explain such an effect by the endogenous circadian rhythm in blood–brain barrier permeability, which shows reduced trafficking during the active phase compared to other periods during the day [154].
The data regarding the protective effects of antiplatelet drugs (in particular, low-dose aspirin) [155] administered depending on the time of the day are rather conflicting [151, 156].
Melatonin Supplementation
Second, the modulation of the circadian rhythm by melatonin supplementation has been widely discussed in the last decade. Acting via MT2 receptors, melatonin provides a number of neuroprotective effects including antioxidant, anti-inflammatory, antiexcitotoxic, antiapoptotic, and other properties [157,158,159]. In animal models, exogenous melatonin reduces the brain lesion size and neural loss [160, 161] and improves functional outcomes [162, 163], which can be related to the decrease in ischemia–reperfusion injury via modulation of the circadian gene expression leading to the phase-delayed expression of PER1, PER2, and Cry1, as well as expression of genes involved in hypoxia-mediated signaling pathways [164, 165]. Moreover, melatonin alleviates circadian disturbances and improves sleep, increasing delta power and decreasing sleep fragmentation in rats after an induced stroke [166]. In clinical settings, melatonin supplementation was associated with a lower risk of post-stroke delirium (a single dose of 2 mg/day at 20:00 within the first 24 h of ischemic stroke onset) compared to the control group [167] and with the shorter duration of mechanical ventilation and length of stay in the intensive care unit in a cohort of patients with hemorrhagic stroke (30 mg melatonin every night during a stay at the intensive care unit) [168]. In early and late rehabilitation periods, melatonin administration (3 mg daily for 3 months) led to an increase in the BDNF level that correlated with improved sleep, emotional status, and quality of life [169, 170]. Regarding effects on functional outcomes, a pilot randomized study showed that 5-day treatment with melatonin in the acute phase of stroke led to a greater reduction in median NIHSS score, but not in mRS at a 3-month follow-up [171]. Ongoing trials aim to investigate both short- and long-term effects of melatonin in patients with stroke: Role of Melatonin in the Acute Phase of Stroke as Measured by Interleukin 6 Biomarker, NCT03843008; The Role of Circadian Factors in Regulation of Neuroplasticity in Ischemic Stroke (Interventional), NCT05247125.
Non-Medication Developments
A number of non-medication interventions, which can reset molecular clocks and modulate circadian rhythms, draw attention as potential neuroprotective strategies, in particular, bright light therapy, physical exercise, and shift in food intake.
Bright Light Therapy
Bright light therapy exhibits various effects including mood improvement (even included as a complementary or alternative treatment for depressive disorders, in particular, for seasonal depression, by various professional associations [172, 173]), increasing alertness, and reduction in fatigue and sleepiness in different cohorts [174]. However, only a few studies evaluated the effects of light in post-stroke survivors, and the evidence on long-term effects is lacking. In a placebo-controlled randomized study, light therapy (10,000 lx, 30 min in the morning) was shown to decrease insomnia symptoms, daytime sleepiness, and fatigue, and improve the emotional state in post-stroke survivors [175]. In post-stroke rehabilitation, naturalistic light in the ward was associated with reduced fatigue, increased levels of melatonin, and present diurnal rhythmicity by discharge, while patients staying at standard indoor light at rehabilitation did not exhibit melatonin diurnal rhythmicity at discharge [176]. However, the study was uncontrolled and no long-term follow-up after discharge was performed. In a study involving patients with past brain injury, including post-stroke subjects in a chronic phase of stroke, bright light therapy was associated with a reduction in subjective sleep quality (sleep disturbance and insomnia symptoms) and improved psychomotor vigilance [177]. More data is expected from the ongoing trials assessing bright light therapy effects in patients with stroke: Role of Melatonin in the Acute Phase of Stroke as Measured by Interleukin 6 Biomarker, NCT03843008; The Role of Circadian Factors in Regulation of Neuroplasticity in Ischemic Stroke (Interventional), NCT05247125; Effects of Colored Light Exposure on Sleep Disturbance, Fatigue, and Functional Outcomes Following Acute Brain Injury, NCT03125967.
Gene Therapy
Last but not least, gene therapy presents a promising neuroprotective approach being investigated in animal models [178,179,180], and clock genes are a candidate potent target.
Conclusion
Despite the increasing evidence of the bidirectional influence of the circadian system and brain damage in stroke, there are a number of gaps in understanding the precise underlying mechanisms. Moreover, the data on the 24-h variation of the main circadian biomarkers and parameters in patients with stroke in acute, subacute, and chronic phases are rather scarce, and their clinical implications and association with the prognosis are not fully understood. The implementation of various approaches and techniques for circadian rhythm entrainment in patients with stroke appears to be a promising complementary treatment for improving post-stroke recovery. However, the criteria to select the subgroups of patients who will benefit most from these interventions are to be established.
References
Lloyd-jones DM, Allen NB, Anderson CAM, et al. Life’s Essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146(5):e18–43. https://doi.org/10.1161/CIR.0000000000001078.
Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–3. https://doi.org/10.1016/j.sleh.2014.12.010.
Knutson KL, Phelan J, Paskow MJ, et al. The National Sleep Foundation’s sleep health index. Sleep Health. 2017;3(4):234–40. https://doi.org/10.1016/j.sleh.2017.05.011.
Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. https://doi.org/10.5665/sleep.3298.
The GBD 2016 Lifetime Risk of Stroke Collaborators. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379:2429–2437. https://doi.org/10.1056/NEJMoa1804492.
Nouh A, Amin-Hanjani S, Furie KL, et al. Identifying best practices to improve evaluation and management of in-hospital stroke: a scientific statement from the American Heart Association. Stroke. 2022;53(4):165–75. https://doi.org/10.1161/STR.0000000000000402.
World Health Organization. Stroke, cerebrovascular accident. WHO reports. https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html. Accessed Feb 2023.
Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29(5):992–6. https://doi.org/10.1161/01.STR.29.5.992.
Fodor DM, Babiciu I, Perju-Dumbrava L. Circadian variation of stroke onset: a hospital-based study. Clujul Med. 2014;87(4):8. https://doi.org/10.15386/cjmed-328.
Fodor DM, Marta MM, Perju-Dumbravă L. Implications of circadian rhythm in stroke occurrence: certainties and possibilities. Brain Sci. 2021;11(7):1–11. https://doi.org/10.3390/brainsci11070865.
Casetta I, Granieri E, Fallica E, Cecilia O, Paolino E, Manfredini R. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol. 2002;59:48–53.
Butt MURA, Zakaria M, Hussain HM. Circadian pattern of onset of ischaemic and haemorrhagic strokes, and their relation to sleep/wake cycle. J Pak Med Assoc. 2009;59(3):129–32.
Rhoney DH, Coplin WM, Lin Y, Frankel M, Lyden PD, Levine SR. Time of day, outcome, and response to thrombolytic therapy: the National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Trial experience. J Stroke Cerebrovasc Dis. 2010;19(1):40–8. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.03.006.
Beker MC, Caglayan B, Yalcin E, et al. Time-of-day dependent neuronal injury after ischemic stroke: implication of circadian clock transcriptional factor Bmal1 and survival kinase AKT. Mol Neurobiol. 2018;55(3):2565–76. https://doi.org/10.1007/s12035-017-0524-4.
Ripamonti L, Riva R, Maioli F, Zenesini C, Procaccianti G. Daily variation in the occurrence of different subtypes of stroke. Stroke Res Treat. 2017;2017:9091250. https://doi.org/10.1155/2017/9091250.
Ryu WS, Hong KS, Jeong SW, et al. Association of ischemic stroke onset time with presenting severity, acute progression, and long-term outcome: a cohort study. PLoS Med. 2022;19(2):1–15. https://doi.org/10.1371/journal.pmed.1003910.
Turin T, Kita Y, Rumana N, et al. Is there any circadian variation consequence on acute case fatality of stroke? Takashima Stroke Registry, Japan (1990–2003). Acta Neurol Scand. 2012;125:206–12. https://doi.org/10.1111/j.1600-0404.2011.01522.x.
Verdecchia P, Angeli F, Mazzotta G, et al. Ambulatory blood pressure monitoring day-night dip and early-morning surge in blood pressure prognostic implications. Hypertension. 2012;60:34–43. https://doi.org/10.1161/HYPERTENSIONAHA.112.191858.
Kario K, Pickering T, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives a prospective study. Circulation. 2003;107:1401–6. https://doi.org/10.1161/01.CIR.0000056521.67546.AA.
Ramsey AM, Stowie A, Castanon-cervantes O, Davidson AJ. Environmental circadian disruption increases stroke severity and dysregulates immune response. J Biol Rhythms. 2020;35(4):368–76. https://doi.org/10.1177/0748730420929450.Environmental.
Earnest D, Neuendorff N, Coffman J, Selvamani A, Sohrabji F. Sex differences in the impact of shift work schedules on pathological outcomes in an animal model of ischemic stroke. Endocrinology. 2016;157(7):2836–43. https://doi.org/10.1210/en.2016-1130.
Earnest DJ, Burns S, Pandey S, Kumar K, Sohrabji F. Sex differences in the diathetic effects of shift work schedules on circulating cytokine levels and pathological outcomes of ischemic stroke during middle age. Neurobiol Sleep Circ Rhythms. 2022;13:100079. https://doi.org/10.1016/j.nbscr.2022.100079.
Mastura K, Mohd K, Wyse C, et al. Chronic photoperiod disruption does not increase vulnerability to focal cerebral ischemia in young normotensive rats. J Cereb Blood Flow Metab. 2017;37(11):3580–8. https://doi.org/10.1177/0271678X16671316.
Cantone L, Tobaldini E, Favero C, et al. Particulate air pollution, clock gene methylation, and stroke: effects on stroke severity and disability. Int J Mol Sci. 2020;21:9. https://doi.org/10.3390/ijms21093090.
Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci. 2016;113(10):E1402–1411.
Yoshizaki T, Ishihara J, Kotemori A, Kokubo Y, Saito I. Association between irregular daily routine and risk of incident stroke and coronary heart disease in a large Japanese population. Sci Rep. 2022;12:1–10. https://doi.org/10.1038/s41598-022-20019-8.
Li M, Huang J, Tan Y, Yang B, Tang Z. Shift work and risk of stroke: a meta-analysis. Int J Cardiol. 2016;214:370–3. https://doi.org/10.1016/j.ijcard.2016.03.052.
Chen Y, Yang L, Liang Y, et al. Interaction of night shift work with polymorphism in melatonin receptor 1B gene on incident stroke. Scand J Work Environ Health. 2022;48(5):372–9. https://doi.org/10.5271/sjweh.4025.
Palatini P, Reboldi G, Beilin LJ, et al. Predictive value of night-time heart rate for cardiovascular events in hypertension. The ABP-international study. Int J Cardiol. 2013;168(2):1490–95. https://doi.org/10.1016/j.ijcard.2012.12.103.
Pickering TG, Schwartz J, Verdecchia P, et al. Prediction of strokes versus cardiac events by ambulatory monitoring of blood pressure: results from an international database. Blood Press Monit. 2007;12(6):397–9. https://doi.org/10.1097/MBP.0b013e3282411a12.
Kario K. Are melatonin and its receptor agonist specific antihypertensive modulators of resistant hypertension caused by disrupted circadian rhythm? J Am Soc Hypertens. 2011;5(5):354–8.
Eguchi K, Kario K, Shimada K, Mori T, Nii T, Ibaragi K. Circadian variation of blood pressure and neurohumoral factors during the acute phase of stroke. Clin Exp Hypertens. 2002;24(1–2):109–14. https://doi.org/10.1081/ceh-100108721.
Dawson SL, Evans SN, Manktelow BN, Fotherby MD, Robinson TG, Potter JF. Diurnal blood pressure change varies with stroke subtype in the acute phase. Stroke. 1998;29:1519–24.
Dawson SL, Manktelow BN, Robinson TG, Panerai RB, Potter JF. Which parameters of beat-to-beat blood pressure and variability best predict early outcome after acute ischemic stroke? 2000;31:463–469.
Jain S, Namboodri KKN, Kumari S, Prabhakar S. Loss of circadian rhythm of blood pressure following acute stroke. BMC Neurol. 2004;4:1–6. https://doi.org/10.1186/1471-2377-4-1.
Castilla-Guerra L, Espino-Montoro A, Fernández-Moreno MC, López-Chozas JM. Abnormal blood pressure circadian rhythm in acute ischaemic stroke: are lacunar strokes really different? Int J Stroke. 2009;4(4):257–61. https://doi.org/10.1111/j.1747-4949.2009.00314.x.
Hickey J, Salmeron E, Lai J. Twenty-four-hour blood pressure variability after acute ischemic stroke. Crit Care Nurs Q. 2002;25(2):1–12.
Ding X, Zhou Y, Pan Y, et al. Dipping pattern and 1-year stroke functional outcome in ischemic stroke or transient ischemic attack. Clin Exp Hypertens. 2023;45:1. https://doi.org/10.1080/10641963.2022.2139384.
Kwon H, Lim J, Kim YS, Moon J, Park H, Kim HY. Cerebral microbleeds are associated with nocturnal reverse dipping in hypertensive patients with ischemic stroke. BMC Neurol. 2014;14:1–8.
Ma JF, Sun JL, Zhao J, Wei X, Wang BS, Fu Y. Relationship between nocturnal blood pressure variation and silent cerebral infarction in Chinese hypertensive patients. J Neurol Sci. 2010;294(1–2):67–9. https://doi.org/10.1016/j.jns.2010.04.002.
Klarenbeek P, Van Oostenbrugge RJ, Rouhl RPW, Knottnerus ILH, Staals J. Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke. 2013;44(11):2995–9. https://doi.org/10.1161/STROKEAHA.113.002545.
Hajjar I, Zhao P, Alsop D, et al. Association of blood pressure elevation and nocturnal dipping with brain atrophy, perfusion and functional measures in stroke and non-stroke individuals. Am J Hypertens. 2010;23(1):17–23. https://doi.org/10.1038/ajh.2009.187.Association.
Sargento-Freitas J, Laranjinha I, Galego O, et al. Nocturnal blood pressure dipping in acute ischemic stroke. Acta Neurol Scand. 2015;132(5):323–8. https://doi.org/10.1111/ane.12402.
Anne M, Juha K, Makikallio T, et al. Neurohormonal activation in ischemic stroke: effects of acute phase disturbances on long-term mortality. Curr Neurovasc Res. 2007;4(3):170–5. https://doi.org/10.2174/156720207781387169.
Scheer F, Shea S. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood. 2014;123(4):590–3. https://doi.org/10.1182/blood-2013-07-517060.
Pinotti M, Bertolucci C, Portaluppi F, Colognesi I, Frigato E, Bernardi F. Daily and circadian rhythms of tissue factor pathway inhibitor and factor VII activity. Arterioscler Thromb Vasc Biol. 2005;25:646–9. https://doi.org/10.1161/01.ATV.0000153140.13148.e0.
Carmona P, Mendez N, Ili CG, Brebi P. The role of clock genes in fibrinolysis regulation: circadian disturbance and its effect on fibrinolytic activity. Front Physiol. 2020;11(March):1–7. https://doi.org/10.3389/fphys.2020.00129.
Cortelli P, Lombardi C, Montagna P, Parati G. Baroreflex modulation during sleep and in obstructive sleep apnea syndrome. Auton Neurosci Basic Clin. 2012;169(1):7–11. https://doi.org/10.1016/j.autneu.2012.02.005.
Legramante JM, Marciani MG, Placidi F, et al. Sleep-related changes in baroreflex sensitivity and cardiovascular autonomic modulation. J Hypertens. 2003;21(8):1555–61. https://doi.org/10.1097/00004872-200308000-00021.
Bassetti CLA, Randerath W, Vignatelli L, Ferini-strambi L, Brill A, Bonsignore MR. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur Respir J. 2019;2020(55):1901104. https://doi.org/10.1183/13993003.01104-2019.
Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):1105–14. https://doi.org/10.5665/sleep.1998.
Elliott R, Nathaney A. Typical sleep patterns are absent in mechanically ventilated patients and their circadian melatonin rhythm is evident but the timing is altered by the ICU environment. Aust Crit Care. 2014;27(3):151–3. https://doi.org/10.1016/j.aucc.2014.01.001.
Lincoln GA, Clarke IJ, Hut RAHD. Characterizing a mammalian circannual pacemaker. Science. 2006;314:1941–4.
Albrecht U. Review timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74(2):246–60. https://doi.org/10.1016/j.neuron.2012.04.006.
Baron KG, Reid KJ, Kim T, et al. Circadian timing and alignment in healthy adults: associations with BMI, body fat, caloric intake and physical activity. Int J Obes. 2017;41(2):203–9. https://doi.org/10.1038/ijo.2016.194.
Viswambharan H, Carvas JM, Antic V, et al. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation. 2007;115(16):2188–95. https://doi.org/10.1161/CIRCULATIONAHA.106.653303.
Carvas JM, Vukolic A, Yepuri G, et al. Period2 gene mutant mice show compromised insulin-mediated endothelial nitric oxide release and altered glucose homeostasis. Front Physiol. 2012;3(August):1–11. https://doi.org/10.3389/fphys.2012.00337.
Korostovtseva L. Ischemic stroke and sleep: the linking genetic factors. Cardiol Ther. 2021;10(2):349–75. https://doi.org/10.1007/s40119-021-00231-9.
Griffin P, Dimitry JM, Sheehan PW, et al. Circadian clock protein Rev-erbα regulates neuroinflammation. PNAS. 2019;116(11):5102–7. https://doi.org/10.1073/pnas.1812405116.
Schallner N, Lieberum J-L, Gallo D, et al. Carbon monoxide preserves circadian rhythm to reduce the severity of subarachnoid hemorrhage in mice. Stroke. 2017;48(9):2565–73. https://doi.org/10.1161/STROKEAHA.116.016165.Carbon.
Zielinski MR, Gibbons AJ. Neuroinflammation, sleep, and circadian rhythms. Front Cell Infect Microbiol. 2022;12(March):1–16. https://doi.org/10.3389/fcimb.2022.853096.
Lembach A, Stahr A, Ali AAH, Ingenwerth M, von Gall C. Sex-dependent effects of Bmal1-deficiency on mouse cerebral cortex infarction in response to photothrombotic stroke. Int J Mol Sci. 2018;19(10):1–16. https://doi.org/10.3390/ijms19103124.
Boden MJ, Varcoe TJ, Voultsios A, Kennaway DJ. Reproductive biology of female Bmal1 null mice. Reproduction. 2010;139(6):1077–90. https://doi.org/10.1530/REP-09-0523.
Tischkau SA, Cohen JA, Stark JT, Gross DR, Bottum KM. Time-of-day affects expression of hippocampal markers for ischemic damage induced by global ischemia. Exp Neurol. 2007;208(2):314–22. https://doi.org/10.1016/j.expneurol.2007.09.003.
Correa-Costa M, Gallo D, Csizmadia E, et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc Natl Acad Sci USA. 2018;115(10):E2302–10. https://doi.org/10.1073/pnas.1716747115.
Corella D, Asensio EM, Coltell O, et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial. Cardiovasc Diabetol. 2016;15(1):1–12. https://doi.org/10.1186/s12933-015-0327-8.
Riestra P, Gebreab SY, Xu R, et al. Circadian CLOCK gene polymorphisms in relation to sleep patterns and obesity in African Americans: findings from the Jackson heart study. BMC Genet. 2017;18(1):1–10. https://doi.org/10.1186/s12863-017-0522-6.
Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, FitzGerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA. 2007;104(9):3450–5. https://doi.org/10.1073/pnas.0611680104.
Masuki S, Todo T, Nakano Y, Okamura H, Nose H. Reduced α-adrenoceptor responsiveness and enhanced baroreflex sensitivity in Cry-deficient mice lacking a biological clock. J Physiol. 2005;566(1):213–24. https://doi.org/10.1113/jphysiol.2005.086728.
Westgate EJ, Cheng Y, Reilly DF, et al. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117(16):2087–95. https://doi.org/10.1161/CIRCULATIONAHA.107.739227.
Schoenhard JA, Smith LH, Painter CA, Eren M, Johnson CH, Vaughan DE. Regulation of the PAI-1 promoter by circadian clock components: differential activation by BMAL1 and BMAL2. J Mol Cell Cardiol. 2003;35(5):473–81. https://doi.org/10.1016/S0022-2828(03)00051-8.
Virag JAI, Dries JL, Easton PR, et al. Attenuation of myocardial injury in mice with functional deletion of the circadian rhythm gene mPer2. Am J Physiol Heart Circ Physiol. 2010;298(3):H1088–95. https://doi.org/10.1152/ajpheart.01280.2008.
Eckle T, Hartmann K, Bonney S, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch critical for myocardial adaptation to ischemia. Nat Med. 2012;18(5):774–82. https://doi.org/10.1038/nm.2728.Adora2b-elicited.
Thackaberry EA, Gabaldon DM, Walker MK, Smith SM. Aryl hydrocarbon receptor null mice develop cardiac hypertrophy and increased hypoxia-inducible factor-1α in the absence of cardiac hypoxia. Cardiovasc Toxicol. 2002;2(4):263–73. https://doi.org/10.1385/CT:2:4:263.
Hernández-Rosas F, López-Rosas CA, Saavedra-Vélez MV. Disruption of the molecular circadian clock and cancer: an epigenetic link. Biochem Genet. 2020;58(1):189–209. https://doi.org/10.1007/s10528-019-09938-w.
Gottlieb E, Churilov L, Werden E, et al. Sleep-wake parameters can be detected in patients with chronic stroke using a multisensor accelerometer: a validation study. J Clin Sleep Med. 2021;17(2):167–75. https://doi.org/10.5664/JCSM.8812.
Campos C, DePaul VG, Knorr S, Wong JS, Mansfield A, Patterson KK. Validity of the ActiGraph activity monitor for individuals who walk slowly post-stroke. Top Stroke Rehabil. 2018;25(4):295–304. https://doi.org/10.1080/10749357.2018.1446487.
Falck RS, Best JR, Li MCR, Eng JJ, Liu-Ambrose T. Revisiting the MotionWatch8©: calibrating cut-points for measuring physical activity and sedentary behavior among adults with stroke. Front Aging Neurosci. 2019;10:1–12. https://doi.org/10.3389/fnagi.2019.00203.
Iacovelli C, Caliandro P, Rabuffetti M, et al. Actigraphic measurement of the upper limbs movements in acute stroke patients. J Neuroeng Rehabil. 2019;16(1):1–10. https://doi.org/10.1186/s12984-019-0603-z.
Gebruers N, Truijen S, Engelborghs S, Nagels G, Brouns R, De Deyn P. Actigraphic measurement of motor deficits in acute ischemic stroke. Cerebrovasc Dis. 2008;26:533–40. https://doi.org/10.1159/000160210.
Kerr G, Sellars C, Bowie L, et al. Xerostomia after acute stroke. Cerebrovasc Dis. 2009;28:624–6. https://doi.org/10.1159/000253147.
Takekawa H, Miyamoto M, Miyamoto T, Yokota N, Hirata K. Alteration of circadian periodicity in core body temperatures of patients with acute stroke. Psychiatry Clin Neurosci. 2002;56(3):221–2. https://doi.org/10.1046/j.1440-1819.2002.00994.x.
Maas MB, Iwanaszko M, Lizza BD, Reid KJ, Braun RI, Zee PC. Circadian gene expression rhythms during critical illness. Crit Care Med. 2020;48(12):e1294–9. https://doi.org/10.1097/CCM.0000000000004697.Circadian.
Barugh AJ, Gray P, Shenkin SD, MacLullich AMJ, Mead GE. Cortisol levels and the severity and outcomes of acute stroke: a systematic review. J Neurol. 2014;261(3):533–45. https://doi.org/10.1007/s00415-013-7231-5.Cortisol.
Al-Ahwal SA, Ragab OA, Elsafa AAA, Ghali AA. Circadian and circannual patterns of stroke. Egypt J Neurol Psychiatry Neurosurg. 2019;55(1):1–7. https://doi.org/10.1186/s41983-019-0051-5.
Zhang XX, Cai XY, Zhao HR, et al. Circadian rhythms of melatonin, cortisol, and clock gene expression in the hyperacute phase of wake-up stroke: study design and measurement. Chin Med J. 2020;133(21):2635–7. https://doi.org/10.1097/CM9.0000000000001111.
Murros K, Fogelholm R, Kettunen S, Vuorela AL. Serum cortisol and outcome of ischemic brain infarction. J Neurol Sci. 1993;116(1):12–7. https://doi.org/10.1016/0022-510X(93)90083-B.
Zhang X, Zou W, Yang Y. Effects of IL-6 and cortisol fluctuations in post-stroke depression. J Huazhong Univ Sci Technol Med Sci. 2016;36(5):732–5. https://doi.org/10.1007/s11596-016-1653-0.
Pang SF, Li Y, Jiang D, Chang B, Xie B. Acute cerebral hemorrhage changes the nocturnal surge of plasma melatonin in humans. J Pineal Res. 1990;9(3):193–208. https://doi.org/10.1111/j.1600-079X.1990.tb00708.x.
Pajediene E, Paulekas E, Salteniene V, et al. Diurnal variation of clock genes expression and other sleep-wake rhythm biomarkers among acute ischemic stroke patients. Sleep Med. 2022;99:1–10. https://doi.org/10.1016/j.sleep.2022.06.023.
Adamczak-Ratajczak A, Kupsz J, Owecki M, et al. Circadian rhythms of melatonin and cortisol in manifest Huntington’s disease and in acute cortical ischemic stroke. J Physiol Pharmacol. 2017;68(4):539–46.
Atanassova PA, Terzieva DD, Dimitrov BD. Impaired nocturnal melatonin in acute phase of ischaemic stroke: cross-sectional matched case-control analysis. J Neuroendocrinol. 2009;21(7):657–63. https://doi.org/10.1111/j.1365-2826.2009.01881.x.
Ritzenthaler T, Nighoghossian N, Berthiller J, et al. Nocturnal urine melatonin and 6-sulphatoxymelatonin excretion at the acute stage of ischaemic stroke. J Pineal Res. 2009;46(3):349–52. https://doi.org/10.1111/j.1600-079X.2009.00670.x.
Fiorina P, Lattuada G, Silvestrini C, Ponari O, Dall AP. Disruption of nocturnal melatonin rhythm and immunological involvement in ischaemic stroke patients. Scand J Immunol. 1999;50(2):228–31. https://doi.org/10.1046/j.1365-3083.1999.00579.x.
Beloosesky Y, Grinblat J, Laudon M, Grosman B, Streifler JY, Zisapel N. Melatonin rhythms in stroke patients. Neurosci Lett. 2002;319(2):103–6. https://doi.org/10.1016/S0304-3940(01)02568-X.
Lorente L, Martín MM, Abreu-González P, et al. Serum melatonin levels are associated with mortality in patients with malignant middle cerebral artery infarction. J Int Med Res. 2018;46(8):3268–77. https://doi.org/10.1177/0300060518775008.
Morin LP, Blanchard JH. Forebrain connections of the hamster intergeniculate leaflet: comparison with those of ventral lateral geniculate nucleus and retina. Vis Neurosci. 1999;16:1037–54.
Meng H, Liu T, Borjigin J, Wang MM. Ischemic stroke destabilizes circadian rhythms. J Circ Rhythms. 2008;6:1–13. https://doi.org/10.1186/1740-3391-6-9.
Zeitzer JM, Ayas NT, Shea SA, Brown R, Czeisler CA, Neuroscience P. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. J Clin Endocrinol Metab. 2015;85(6):2189–96.
Lu HY, Huang APH, Kuo LT. Prognostic value of circadian brain temperature rhythm in basal ganglia hemorrhage after surgery. Neurol Ther. 2021;10(2):1045–59. https://doi.org/10.1007/s40120-021-00283-y.
Ayalon L, Borodkin K, Dishon L, Kanety H, Dagan Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 2007;68(14):1136–40. https://doi.org/10.1212/01.wnl.0000258672.52836.30.
Takekawa H, Miyamoto M, Miyamoto T, Hirata K. Circadian rhythm abnormalities in the acute phase of cerebral infarction correlate with poor prognosis in the chronic phase. Auton Neurosci Basic Clin. 2007;131(1–2):131–6. https://doi.org/10.1016/j.autneu.2006.08.008.
Mackenzie MA, Schonbaum E, Hermus ARMM, et al. Sudornotor function in human poikilothermia. Neurology. 1995;45(August):1602–8.
Pizza F, Vetrugno R, Antelmi E, Pierangeli G, Montagna P, Cortelli P. Narcoleptic-like hypersomnia and inverted circadian rhythm of body core temperature after traumatic brain injury involving the hypothalamus. Sleep Med. 2011;12(10):1044–5. https://doi.org/10.1016/j.sleep.2011.06.013.
Geurts M, Scheijmans FEV, van Seeters T, et al. Temporal profile of body temperature in acute ischemic stroke: relation to infarct size and outcome. BMC Neurol. 2016;16(1):1–7. https://doi.org/10.1186/s12883-016-0760-7.
Karaszewski B, Thomas RGR, Dennis MS, Wardlaw JM. Temporal profile of body temperature in acute ischemic stroke: relation to stroke severity and outcome. BMC Neurol. 2012;12:6–15.
Saini M, Saqqur M, Kamruzzaman A, Lees KR, Shuaib A. Effect of hyperthermia on prognosis after acute ischemic stroke. Stroke. 2009;40(9):3051–9. https://doi.org/10.1161/STROKEAHA.109.556134.
Boysen G, Christensen H. Stroke severity determines body temperature in acute stroke. Stroke. 2001;32(2):413–7. https://doi.org/10.1161/01.STR.32.2.413.
Kvistad CE, Thomassen L, Waje-Andreassen U, Naess H. Low body temperature associated with severe ischemic stroke within 6 hours of onset: the Bergen NORSTROKE Study. Vasc Health Risk Manag. 2012;8(1):333–8. https://doi.org/10.2147/VHRM.S31614.
Wang Y, Lim LLY, Levi C, Heller RF, Fisher J. Influence of admission body temperature on stroke mortality. Stroke. 2000;31:404–9. https://doi.org/10.1053/jscd.2001.20976.
Ruborg R, Gunnarsson K, Ström JO. Predictors of post-stroke body temperature elevation. BMC Neurol. 2017;17(1):1–7. https://doi.org/10.1186/s12883-017-1002-3.
Teicher MH, Lawrence JM, Bakber NI, Finklestein SP, Lieberman H, Baldessakini RJ. Altered locomotor activity in neuropsychiatric patients. Progr Neuropsychopharmacol Biol Psychiatry. 1986;10(6):755–61. https://doi.org/10.1016/0278-5846(86)90061-8.
Shevelev O, Petrova M, Yuriev M, Mengistu E. Circadian temperature rhythms of the healthy and damaged brain. J Neurosci Neurol Disord. 2022;6:032–3. https://doi.org/10.1080/02699050010007515.
Lee JY, Kwon SY, Kim WS, Hahn SJ, Park J, Paik NJ. Feasibility, reliability, and validity of using accelerometers to measure physical activities of patients with stroke during inpatient rehabilitation. PLoS ONE. 2018;13(12):1–13. https://doi.org/10.1371/journal.pone.0209607.
Reiterer V, Sauter C, Klösch G, Lalouschek W, Zeitlhofer J. Actigraphy—a useful tool for motor activity monitoring in stroke patients. Eur Neurol. 2008;60(6):285–91. https://doi.org/10.1159/000157882.
English C, Healy GN, Coates A, Lewis L, Olds T. Sitting and acitivty time in people with stroke. Phys Ther. 2016;96(2):193–201.
Shepherd AI, Pulsford R, Poltawski L, et al. Physical activity, sleep, and fatigue in community dwelling stroke survivors. Sci Rep. 2018;8(1):1–8. https://doi.org/10.1038/s41598-018-26279-7.
Bakken LN, Lee KA, Kim HS, Finset A, Lerdal A. Sleep-wake patterns during the acute phase after first-ever stroke. Stroke Res Treat. 2011;2011(936298):1–7. https://doi.org/10.4061/2011/936298.
Williams-Cooke C, Watts E, Bonnett J, Alshehri M, Siengsukon C. Association between sleep duration and functional disability in inpatient stroke rehabilitation: a pilot observational study. Arch Rehabil Res Clin Transl. 2021;3(3):100150. https://doi.org/10.1016/j.arrct.2021.100150.
Fleming MK, Smejka T, Henderson Slater D, Chiu EG, Demeyere N, Johansen-Berg H. Self-reported and objective sleep measures in stroke survivors with incomplete motor recovery at the chronic stage. Neurorehabil Neural Repair. 2021;35(10):851–60. https://doi.org/10.1177/15459683211029889.
Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiat. 1990;27(6):563–72. https://doi.org/10.1016/0006-3223(90)90523-5.
Gonçalves BSB, Cavalcanti PRA, Tavares GR, Campos TF, Araujo JF. Nonparametric methods in actigraphy: an update. Sleep Sci. 2014;7(3):158–64. https://doi.org/10.1016/j.slsci.2014.09.013.
Sommer R, Yu L, Schneider JA, Bennett DA, Buchman AS, Lim ASP. Disrupted rest-activity rhythms and cerebral small vessel disease pathology in older adults. Stroke. 2021;2:2427–31. https://doi.org/10.1161/STROKEAHA.120.030870.
Zuurbier LA, Ikram MA, Luik AI, et al. Cerebral small vessel disease is related to disturbed 24-h activity rhythms: a population-based study. Eur J Neurol. 2015;22(11):1482–7. https://doi.org/10.1111/ene.12775.
Reale G, Giovannini S, Iacovelli C, et al. Actigraphic measurement of the upper limbs for the prediction of ischemic stroke prognosis: an observational study. Sensors. 2021;21(7):1–10. https://doi.org/10.3390/s21072479.
Maas MB, Lizza BD, Abbott SM, et al. Factors disrupting melatonin secretion rhythms during critical illness. Crit Care Med. 2020;48(6):854–61. https://doi.org/10.1097/CCM.0000000000004333.Factors.
Maas MB, Lizza BD, Kim M, et al. Stress-induced behavioral quiescence and abnormal rest-activity rhythms during critical illness. Crit Care Med. 2021;48(6):862–71. https://doi.org/10.1097/CCM.0000000000004334.Stress-Induced.
Xu S, Lu J, Shao A, Zhang JH, Zhang J. Glial cells: role of the immune response in ischemic stroke. Front Immunol. 2020;11(February):1–16. https://doi.org/10.3389/fimmu.2020.00294.
Filchenko I, Korostovtseva L, Bochkarev M, Sviryaev Y. Brain damage in sleep-disordered breathing: the role of glia. Zh Nevrol Psikhiatr Im S S Korsakova. 2022;122(1):15–22. https://doi.org/10.17116/jnevro202212201115.
Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40(3):S8-12. https://doi.org/10.1161/STROKEAHA.108.533166.Astrocytes.
Ali AAH, Schwarz-herzke B, Rollenhagen A, et al. Bmal1-deficiency affects glial synaptic coverage of the hippocampal mossy fiber synapse and the actin cytoskeleton in astrocytes. Glia. 2019. https://doi.org/10.1002/glia.23754.
Karmarkar SW, Tischkau SA. Influences of the circadian clock on neuronal susceptibility to excitotoxicity. Front Physiol. 2013;4:1–11. https://doi.org/10.3389/fphys.2013.00313.
Chi-castañeda D, Ortega A. Circadian regulation of glutamate transporters. Front Endocrinol. 2018;9(June):1–6. https://doi.org/10.3389/fendo.2018.00340.
Vieira E, Mirizio GG, Barin GR, Fawzi N, Nimer S, La SL. Clock genes, inflammation and the immune system—implications for diabetes, obesity and neurodegenerative diseases. Int J Mol Sci. 2020;21:1–8.
Wang X, Li L. Circadian clock regulates in flammation and the development of neurodegeneration. Front Cell Infect Microbiol. 2021;11(September):1–16. https://doi.org/10.3389/fcimb.2021.696554.
Zhang Y, Liu L, Zhao X, Yan S, Zeng F, Zhou D. New insight into ischemic stroke: circadian rhythm in post-stroke angiogenesis. Front Pharmacol. 2022;13(August):1–16. https://doi.org/10.3389/fphar.2022.927506.
Tsuzuki K, Shimizu Y, Suzuki J, et al. Adverse effect of circadian rhythm disorder on reparative angiogenesis in hind limb ischemia. J Am Heart Assoc. 2021;10(16):e020896. https://doi.org/10.1161/JAHA.121.020896.
Xu L, Liu Y, Cheng Q, Shen Y, Yuan Y, Jiang X. Bmal1 downregulation worsens critical limb ischemia by promoting inflammation and impairing angiogenesis. Front Cardiovasc Med. 2021;8(August):1–16. https://doi.org/10.3389/fcvm.2021.712903.
Matsuo R, Ago T, Kamouchi M, et al. Clinical significance of plasma VEGF value in ischemic stroke—research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol. 2013;13:1–8.
Walker W 2nd, Borniger J, Gaudier-Diaz M, et al. Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Mol Psychiatry. 2020;25(5):1080–93. https://doi.org/10.1038/s41380-019-0430-4.Acute.
Sengupta S, Yang G, Donnell JCO, et al. The circadian gene Rev-erbα improves cellular bioenergetics and provides preconditioning for protection against oxidative stress. Free Radic Biol Med. 2016;93:177–89. https://doi.org/10.1016/j.freeradbiomed.2016.02.004.The.
Wolff SEC, Wang XL, Jiao H, et al. The effect of Rev-erbα agonist SR9011 on the immune response and cell metabolism of microglia. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.550145.
Zang M, Zhao Y, Gao L, et al. The circadian nuclear receptor RORα negatively regulates cerebral ischemia-reperfusion injury and mediates the neuroprotective effects of melatonin. BBA Mol Basis Dis. 2020. https://doi.org/10.1016/j.bbadis.2020.165890.
Cain SW, Chang A, Vlasac I, et al. Circadian rhythms in plasma brain-derived neurotrophic factor differ in men and women. J Biol Rhythms. 2017;32(1):75–82. https://doi.org/10.1177/0748730417693124.
Liang F, Walline R, Earnest DJ. Circadian rhythm of brain-derived neurotrophic factor in the rat suprachiasmatic nucleus. Neurosci Lett. 1998;242:89–92.
Girardet C, Lebrun B, Cabirol-Pol M-J, et al. Brain-derived neurotrophic factor/TrkB signaling regulates daily astroglial plasticity in the suprachiasmatic nucleus: electron-microscopic evidence in mouse. Glia. 2013;61:1172–7. https://doi.org/10.1002/glia.22509.
Liang F, Allen G, Earnest D. Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light. J Neurosci. 2000;20(8):2978–87.
Smolensky M, Hermida R, Geng Y-J. Chronotherapy of cardiac and vascular disease: timing medications to circadian rhythms to optimize treatment effects and outcomes. Curr Opin Pharmacol. 2020;57:41–8. https://doi.org/10.1016/j.coph.2020.10.014.
Mackenzie IS, Rogers A, Poulter NR, et al. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet. 2022;400(10361):1417–25. https://doi.org/10.1016/S0140-6736(22)01786-X.
Stergiou G, Brunström M, MacDonald T, et al. Bedtime dosing of antihypertensive medications: systematic review and consensus statement: International Society of Hypertension position paper endorsed by World Hypertension League and European Society of Hypertension. J hypertens. 2022;40(10):1847–58. https://doi.org/10.1097/HJH.0000000000003240.
Bonten TN, Snoep JD, Assendelft WJJ, et al. Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: a randomized cross-over trial. Hypertension. 2015;65(4):743–50. https://doi.org/10.1161/HYPERTENSIONAHA.114.04980.
Brunner-Ziegler S, Jilma B, Schörgenhofer C, et al. Comparison between the impact of morning and evening doses of rivaroxaban on the circadian endogenous coagulation rhythm in healthy subjects. J Thromb Haemost. 2016;14(2):316–23. https://doi.org/10.1111/jth.13213.
Vilas D, Gomis M, Blanco M, et al. Circadian rhythms in the efficacy of intravenous alteplase in patients with acute ischemic stroke and middle cerebral artery occlusion. Chronobiol Int. 2012;29(10):1383–9. https://doi.org/10.3109/07420528.2012.728655.
Liu JA, Walton JC, DeVries AC, Nelson RJ. Disruptions of circadian rhythms and thrombolytic therapy during ischemic stroke intervention. Front Neurosci. 2021;15(June):1–15. https://doi.org/10.3389/fnins.2021.675732.
Miciak-Lawicka E, Begier-Krasinska B, Tykarski A, Krasinski Z. Does the timing of aspirin administration influence its antiplatelet effect—review of literature on chronotherapy. Kardiochir Torakochirurgia Pol. 2018;15(2):125–9. https://doi.org/10.5114/kitp.2018.76479.
Snoep JD, Hovens MMC, Pasha SM, et al. Time-dependent effects of low-dose aspirin on plasma renin activity, aldosterone, cortisol, and catecholamines. Hypertension. 2009;54:1136–43. https://doi.org/10.1161/HYPERTENSIONAHA.109.134825.
Andrabi SS, Parvez S, Tabassum H. Melatonin and ischemic stroke: mechanistic roles and action. Adv Pharmacol Sci. 2015. https://doi.org/10.1155/2015/384750.
Shinozuka K, Staples M, Borlongan CV. Melatonin-based therapeutics for neuroprotection in stroke. Int J Mol Sci. 2013;14(5):8924–47. https://doi.org/10.3390/ijms14058924.
Chern CM, Liao JF, Wang YH, Shen YC. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radical Biol Med. 2012;52(9):1634–47. https://doi.org/10.1016/j.freeradbiomed.2012.01.030.
Liu ZJ, Ran YY, Qie SY, et al. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther. 2019;25(12):1353–62. https://doi.org/10.1111/cns.13261.
Ran Y, Ye L, Ding Z, et al. Melatonin protects against ischemic brain injury by modulating PI3K/AKT signaling pathway via suppression of PTEN activity. ASN Neuro. 2021;13:1–16. https://doi.org/10.1177/17590914211022888.
Wei N, Pu Y, Yang Z, Pan Y, Liu L. Therapeutic effects of melatonin on cerebral ischemia reperfusion injury: role of Yap-OPA1 signaling pathway and mitochondrial fusion. Biomed Pharmacother. 2019;110(119):203–12. https://doi.org/10.1016/j.biopha.2018.11.060.
Cai H, Liang J, Liu Z, et al. Causal effects of sleep traits on ischemic stroke and its subtypes: a Mendelian randomization study. Nat Sci Sleep. 2020;12:783–90. https://doi.org/10.2147/NSS.S265946.
Rhim T, Lee DY, Lee M. Hypoxia as a target for tissue specific gene therapy. J Control Release. 2013;172(2):484–94. https://doi.org/10.1016/j.jconrel.2013.05.021.
Jiménez-Ortega V, Barquilla PC, Pagano ES, Fernández-Mateos P, Esquifino AI, Cardinali DP. Melatonin supplementation decreases prolactin synthesis and release in rat adenohypophysis: correlation with anterior pituitary redox state and circadian clock mechanisms. Chronobiol Int. 2012;29(8):1021–35. https://doi.org/10.3109/07420528.2012.705936.
Hao SM, Zhong ZG, Qu WM, Huang ZL, Sun FY, Qiu MH. Melatonin supplementation in the subacute phase after ischemia alleviates postischemic sleep disturbances in rats. Brain Behav. 2021;11(10):1–10. https://doi.org/10.1002/brb3.2366.
Mengel A, Zurloh J, Boßelmann C, et al. Delirium reduction after administration of melatonin in acute ischemic stroke (DREAMS): a propensity score–matched analysis. Eur J Neurol. 2021;28(6):1958–66. https://doi.org/10.1111/ene.14792.
Dianatkhah M, Najafi A, Sharifzadeh M, et al. Melatonin supplementation may improve the outcome of patients with hemorrhagic stroke in the intensive care unit. J Res Pharmacy Pract. 2017;6(3):173. https://doi.org/10.4103/jrpp.jrpp_17_49.
Milman U, Blum S, Shapira C, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2–2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28(2):341–7. https://doi.org/10.1161/ATVBAHA.107.153965.
Kostenko E. [Influence chronopharmacology therapy methionine (melaxen) on the dynamics of sleep disturbance, cognitive and emotional disorders, brain-derived neurotrophic factor (BDNF) in patients with cerebral stroke in the early and late recovery periods] [In Russian]. Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117(3):56–64. https://doi.org/10.17116/jnevro20171173156-64.
Mehrpooya M, Mazdeh M, Rahmani E. Melatonin supplementation may benefit patients with acute ischemic stroke not eligible for reperfusion therapies: results of a pilot study. J Clin Neurosci. 2022;106:66–75. https://doi.org/10.1016/j.jocn.2022.10.006.
NICE. Depression in adults: treatment and management, NICE guideline. NICE Guideline. 2022;(June):1–113. www.nice.org.uk/guidance/ng222.
American Psychological Association. Clinical practice guideline for the treatment of depression across three age cohorts. 2019. 86 p. Retrieved from https://www.apa.org/depression-guideline. Accessed Feb 2023
O’Brien K, Zizzi H, Brown L, et al. Randomized trial of light therapy for cognitive function during inpatient rehabilitation for acquired brain injury. Arch Phys Med Rehabil. 2017;98(12):e173–4. https://doi.org/10.1016/j.apmr.2017.09.080.
Kim W, Joa K, Kim C, et al. The effect of bright light therapy on sleep and quality of life in patients with poststroke insomnia. Psychosom Med. 2022;84(1):123–30. https://doi.org/10.1097/PSY.0000000000001014.
West A, Simonsen SA, Jennum P, et al. An exploratory investigation of the effect of naturalistic light on fatigue and subjective sleep quality in stroke patients admitted for rehabilitation: a randomized controlled trial. NeuroRehabilitation. 2019;45(2):187–200. https://doi.org/10.3233/NRE-192752.
Connolly LJ, Rajaratnam SMW, Murray JM, Spitz G, Lockley SW, Ponsford JL. Home-based light therapy for fatigue following acquired brain injury: a pilot randomized controlled trial. BMC Neurol. 2021;21(1):1–13. https://doi.org/10.1186/s12883-021-02292-8.
Hyun H, Lee J, Hwang DW, et al. Combinational therapy of ischemic brain stroke by delivery of heme oxygenase-1 gene and dexamethasone. Biomaterials. 2011;32(1):306–15. https://doi.org/10.1016/j.biomaterials.2010.08.116.
Lee Y, Lee J, Kim M, Kim GY, Choi JS, Lee M. Brain gene delivery using histidine and arginine-modified dendrimers for ischemic stroke therapy. J Control Release. 2021;330(October):907–19. https://doi.org/10.1016/j.jconrel.2020.10.064.
Oh J, Lee MS, Jeong JH, Lee M. Deoxycholic acid-conjugated polyethylenimine for delivery of heme oxygenase-1 gene in rat ischemic stroke model. J Pharm Sci. 2017;106(12):3524–32. https://doi.org/10.1016/j.xphs.2017.07.020.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Lyudmila Korostovtseva: concept, draft preparation, revision; Sergey Kolomeichuk: revision, final approval of the text.
Disclosures
Lyudmila S Korostovtseva and Sergey N Kolomeichuk have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Korostovtseva, L.S., Kolomeichuk, S.N. Circadian Factors in Stroke: A Clinician’s Perspective. Cardiol Ther 12, 275–295 (2023). https://doi.org/10.1007/s40119-023-00313-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-023-00313-w