Abstract
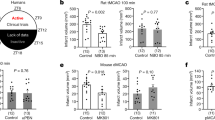
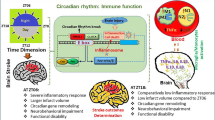
Occurrence of stroke cases displays a time-of-day variation in human. However, the mechanism linking circadian rhythm to the internal response mechanisms against pathophysiological events after ischemic stroke remained largely unknown. To this end, temporal changes in the susceptibility to ischemia/reperfusion (I/R) injury were investigated in mice in which the ischemic stroke induced at four different Zeitgeber time points with 6-h intervals (ZT0, ZT6, ZT12, and ZT18). Besides infarct volume and brain swelling, neuronal survival, apoptosis, ischemia, and circadian rhythm related proteins were examined using immunohistochemistry, Western blot, planar surface immune assay, and liquid chromatography–mass spectrometry tools. Here, we present evidence that midnight (ZT18; 24:00) I/R injury in mice resulted in significantly improved infarct volume, brain swelling, neurological deficit score, neuronal survival, and decreased apoptotic cell death compared with ischemia induced at other time points, which were associated with increased expressions of circadian proteins Bmal1, PerI, and Clock proteins and survival kinases AKT and Erk-1/2. Moreover, ribosomal protein S6, mTOR, and Bad were also significantly increased, while the levels of PRAS40, negative regulator of AKT and mTOR, and phosphorylated p53 were decreased at this time point compared to ZT0 (06:00). Furthermore, detailed proteomic analysis revealed significantly decreased CSKP, HBB-1/2, and HBA levels, while increased GNAZ, NEGR1, IMPCT, and PDE1B at midnight as compared with early morning. Our results indicate that nighttime I/R injury results in less severe neuronal damage, with increased neuronal survival, increased levels of survival kinases and circadian clock proteins, and also alters the circadian-related proteins.





Similar content being viewed by others
References
Roenneberg T, Merrow M (2002) "What watch?...such much!" Complexity and evolution of circadian clocks. Cell Tissue Res 309(1):3–9. doi:10.1007/s00441-002-0568-1
Paranjpe DA, Sharma VK (2005) Evolution of temporal order in living organisms. J Circadian Rhythms 3(1):7. doi:10.1186/1740-3391-3-7
Fodor DM, Babiciu I, Perju-Dumbrava L (2014) Circadian variation of stroke onset: a hospital-based study. Clujul medical 87(4):242–249. doi:10.15386/cjmed-328
Chang AM, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, Lockley SW, Kronauer RE et al (2012) Human responses to bright light of different durations. J Physiol 590(13):3103–3112. doi:10.1113/jphysiol.2011.226555
Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS (2011) Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A 108(4):1657–1662. doi:10.1073/pnas.1018375108
Khapre RV, Kondratova AA, Susova O, Kondratov RV (2011) Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle 10(23):4162–4169. doi:10.4161/cc.10.23.18381
Pardiwalla FK, Yeolekar ME, Bakshi SK (1993) Circadian rhythm in acute stroke. J Assoc Physicians India 41(4):203–204
Elliott WJ (1998) Circadian variation in the timing of stroke onset: a meta-analysis. Stroke 29(5):992–996
Razorenova OV (2012) Brain and muscle ARNT-like protein BMAL1 regulates ROS homeostasis and senescence: a possible link to hypoxia-inducible factor-mediated pathway. Cell Cycle 11(2):213–214. doi:10.4161/cc.11.2.18786
Manev H, Uz T (1998) The role of the light-dark cycle and melatonin in stroke outcome. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 7(3):165–167
Beker MC, Caglayan AB, Kelestemur T, Caglayan B, Yalcin E, Yulug B, Kilic U, Hermann DM et al (2015) Effects of normobaric oxygen and melatonin on reperfusion injury: role of cerebral microcirculation. Oncotarget 6(31):30604–30614. doi:10.18632/oncotarget.5773
Wisniewski JR, Zougman A, Nagaraj N, Mann M (2009) Universal sample preparation method for proteome analysis. Nat Methods 6(5):359–362. doi:10.1038/nmeth.1322
Hacariz O, Baykal AT, Akgun M, Kavak P, Sagiroglu MS, Sayers GP (2014) Generating a detailed protein profile of Fasciola hepatica during the chronic stage of infection in cattle. Proteomics 14(12):1519–1530. doi:10.1002/pmic.201400012
Serhatli M, Baysal K, Acilan C, Tuncer E, Bekpinar S, Baykal AT (2014) Proteomic study of the microdissected aortic media in human thoracic aortic aneurysms. J Proteome Res 13(11):5071–5080. doi:10.1021/pr5006586
Acioglu C, Mirabelli E, Baykal AT, Ni L, Ratnayake A, Heary RF, Elkabes S (2016) Toll like receptor 9 antagonism modulates spinal cord neuronal function and survival: direct versus astrocyte-mediated mechanisms. Brain Behav Immun 56:310–324. doi:10.1016/j.bbi.2016.03.027
Zheng X, Sehgal A (2010) AKT and TOR signaling set the pace of the circadian pacemaker. Current biology : CB 20(13):1203–1208. doi:10.1016/j.cub.2010.05.027
Ko ML, Jian K, Shi L, Ko GY (2009) Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. J Neurochem 108(6):1607–1620. doi:10.1111/j.1471-4159.2009.05931.x
Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM (2006) The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF’s neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 20(8):1185–1187. doi:10.1096/fj.05-4829fje
Spudich A, Kilic E, Xing H, Kilic U, Rentsch KM, Wunderli-Allenspach H, Bassetti CL, Hermann DM (2006) Inhibition of multidrug resistance transporter-1 facilitates neuroprotective therapies after focal cerebral ischemia. Nat Neurosci 9(4):487–488
Turin TC, Kita Y, Rumana N, Nakamura Y, Takashima N, Ichikawa M, Sugihara H, Morita Y et al (2012) Is there any circadian variation consequence on acute case fatality of stroke? Takashima Stroke Registry, Japan (1990-2003). Acta Neurol Scand 125(3):206–212. doi:10.1111/j.1600-0404.2011.01522.x
Kubota K, Sakurai T, Tamura J, Shirakura T (1987) Is the circadian change in hematocrit and blood viscosity a factor triggering cerebral and myocardial infarction? Stroke 18(4):812–813
Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ et al (2015) Beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 18(7):1051–1057. doi:10.1038/nn.4035
Zunzunegui C, Gao B, Cam E, Hodor A, Bassetti CL (2011) Sleep disturbance impairs stroke recovery in the rat. Sleep 34(9):1261–1269. doi:10.5665/SLEEP.1252
Soddu A, Bassetti CL (2017) A good sleep for a fresh mind in patients with acute traumatic brain injury. Neurology 88(3):226–227. doi:10.1212/WNL.0000000000003529
Kunz A, Dirnagl U, Mergenthaler P (2010) Acute pathophysiological processes after ischaemic and traumatic brain injury. Best Pract Res Clin Anaesthesiol 24(4):495–509. doi:10.1016/j.bpa.2010.10.001
Hermann DM, Kilic E, Hata R, Hossmann KA, Mies G (2001) Relationship between metabolic dysfunctions, gene responses and delayed cell death after mild focal cerebral ischemia in mice. Neuroscience 104(4):947–955
Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ et al (2009) Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain : a journal of neurology 132(Pt 8):2239–2251. doi:10.1093/brain/awp174
Vinall PE, Kramer MS, Heinel LA, Rosenwasser RH (2000) Temporal changes in sensitivity of rats to cerebral ischemic insult. J Neurosurg 93(1):82–89. doi:10.3171/jns.2000.93.1.0082
Tischkau SA, Cohen JA, Stark JT, Gross DR, Bottum KM (2007) Time-of-day affects expression of hippocampal markers for ischemic damage induced by global ischemia. Exp Neurol 208(2):314–322. doi:10.1016/j.expneurol.2007.09.003
Okamura H, Yamaguchi S, Yagita K (2002) Molecular machinery of the circadian clock in mammals. Cell Tissue Res 309(1):47–56. doi:10.1007/s00441-002-0572-5
Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD (2002) Circadian rhythms in isolated brain regions. The Journal of neuroscience : the official journal of the Society for Neuroscience 22(1):350–356
Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T (2004) Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 5:18. doi:10.1186/1471-2199-5-18
Fahrenkrug J, Hannibal J, Georg B (2008) Diurnal rhythmicity of the canonical clock genes Per1, Per2 and Bmal1 in the rat adrenal gland is unaltered after hypophysectomy. J Neuroendocrinol 20(3):323–329. doi:10.1111/j.1365-2826.2008.01651.x
Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP (2009) Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging 1(12):979–987. doi:10.18632/aging.100113
Sharp FR, Bergeron M, Bernaudin M (2001) Hypoxia-inducible factor in brain. Adv Exp Med Biol 502:273–291
Pore N, Jiang Z, Shu HK, Bernhard E, Kao GD, Maity A (2006) Akt1 activation can augment hypoxia-inducible factor-1alpha expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Molecular cancer research : MCR 4(7):471–479. doi:10.1158/1541-7786.MCR-05-0234
Meng H, Liu T, Borjigin J, Wang MM (2008) Ischemic stroke destabilizes circadian rhythms. J Circadian Rhythms 6:9. doi:10.1186/1740-3391-6-9
Boone DR, Sell SL, Micci MA, Crookshanks JM, Parsley M, Uchida T, Prough DS, DeWitt DS et al (2012) Traumatic brain injury-induced dysregulation of the circadian clock. PLoS One 7(10):e46204. doi:10.1371/journal.pone.0046204
Kilic E, ElAli A, Kilic U, Guo Z, Ugur M, Uslu U, Bassetti CL, Schwab ME et al (2010) Role of Nogo-A in neuronal survival in the reperfused ischemic brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 30(5):969–984. doi:10.1038/jcbfm.2009.268
Yan J, Wang H, Liu Y, Shao C (2008) Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput Biol 4(10):e1000193. doi:10.1371/journal.pcbi.1000193
Kohsaka A, Bass J (2007) A sense of time: how molecular clocks organize metabolism. Trends in endocrinology and metabolism: TEM 18(1):4–11. doi:10.1016/j.tem.2006.11.005
Koh PO (2012) Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation. Neurosci Lett 507(2):156–160. doi:10.1016/j.neulet.2011.12.012
Hata Y, Butz S, Sudhof TC (1996) CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. The Journal of neuroscience : the official journal of the Society for Neuroscience 16(8):2488–2494
Nafzger S, Rougier JS (2016) Calcium/calmodulin-dependent serine protein kinase CASK modulates the L-type calcium current. Cell Calcium. doi:10.1016/j.ceca.2016.10.001
Bele T, Fabbretti E (2016) The scaffold protein calcium/calmodulin-dependent serine protein kinase controls ATP release in sensory ganglia upon P2X3 receptor activation and is part of an ATP keeper complex. J Neurochem 138(4):587–597. doi:10.1111/jnc.13680
Srivastava S, McMillan R, Willis J, Clark H, Chavan V, Liang C, Zhang H, Hulver M et al (2016) X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner. Acta neuropathologica communications 4:30. doi:10.1186/s40478-016-0295-6
Wong YH, Conklin BR, Bourne HR (1992) Gz-mediated hormonal inhibition of cyclic AMP accumulation. Science 255(5042):339–342
Ho MK, Wong YH (2001) G(z) signaling: emerging divergence from G(i) signaling. Oncogene 20(13):1615–1625. doi:10.1038/sj.onc.1204190
Hultman R, Kumari U, Michel N, Casey PJ (2014) Galphaz regulates BDNF-induction of axon growth in cortical neurons. Mol Cell Neurosci 58:53–61. doi:10.1016/j.mcn.2013.12.004
Roffe M, Hajj GN, Azevedo HF, Alves VS, Castilho BA (2013) IMPACT is a developmentally regulated protein in neurons that opposes the eukaryotic initiation factor 2alpha kinase GCN2 in the modulation of neurite outgrowth. J Biol Chem 288(15):10860–10869. doi:10.1074/jbc.M113.461970
Sattlegger E, Barbosa JA, Moraes MC, Martins RM, Hinnebusch AG, Castilho BA (2011) Gcn1 and actin binding to Yih1: implications for activation of the eIF2 kinase GCN2. J Biol Chem 286(12):10341–10355. doi:10.1074/jbc.M110.171587
Dlaboga D, Hajjhussein H, O'Donnell JM (2008) Chronic haloperidol and clozapine produce different patterns of effects on phosphodiesterase-1B, -4B, and -10A expression in rat striatum. Neuropharmacology 54(4):745–754. doi:10.1016/j.neuropharm.2007.12.002
Kim H, Chun Y, Che L, Kim J, Lee S, Lee S (2017) The new obesity-associated protein, neuronal growth regulator 1 (NEGR1), is implicated in Niemann-Pick disease Type C (NPC2)-mediated cholesterol trafficking. Biochem Biophys Res Commun 482(4):1367–1374. doi:10.1016/j.bbrc.2016.12.043
He Y, Hua Y, Lee JY, Liu W, Keep RF, Wang MM, Xi G (2010) Brain alpha- and beta-globin expression after intracerebral hemorrhage. Translational stroke research 1(1):48–56. doi:10.1007/s12975-009-0004-x
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. The Lancet Neurology 5(1):53–63. doi:10.1016/S1474-4422(05)70283-0
Acknowledgements
This study was supported by The Turkish Academy of Sciences (TUBA) and Necmettin Erbakan University (Scientific Research Project: 161330001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study has been conducted in accordance with the ethical standards and according to the Declaration of Helsinki and according to national and international guidelines and has been approved by the Ethics Committee of Istanbul Medipol University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Beker, M.C., Caglayan, B., Yalcin, E. et al. Time-of-Day Dependent Neuronal Injury After Ischemic Stroke: Implication of Circadian Clock Transcriptional Factor Bmal1 and Survival Kinase AKT. Mol Neurobiol 55, 2565–2576 (2018). https://doi.org/10.1007/s12035-017-0524-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0524-4