Abstract
Introduction
Trimetazidine (TMZ) is an anti-ischemic metabolic agent that has been shown to be efficacious in angina treatment, both in monotherapy and in combination. A new formulation of TMZ modified-release (MR) 80 mg was developed, which is to be taken once daily (od), instead of twice daily (bid) for the currently available TMZ MR 35 mg, with the aim of simplifying the medication regimen.
Methods
The present study was an international, multicenter, randomized, double-blind, parallel-group phase III study with a 12-week treatment period. The safety of TMZ MR 80 mg once daily was assessed compared to TMZ MR 35 mg twice daily, in patients previously treated successfully by the latter. Emergent adverse events (EAEs), biological parameters, vital signs, 12-lead resting ECG (electrocardiogram) and Canadian Cardiovascular Society (CCS) classification were recorded.
Results
One-hundred and sixty-five patients previously diagnosed with stable angina pectoris on treatment were randomized to receive either TMZ MR 80 mg od or TMZ MR 35 mg bid. In the TMZ MR 80 mg group, fewer patients had ≥ 1 EAE (17.1 vs. 22.9% in the TMZ MR 35 mg group). Serious EAEs were reported by three patients in each group. No EAE required dose modification, withdrawal, or temporary interruption of study treatments. Vital signs, 12-lead ECG, and laboratory parameters did not change. No worsening in CCS classes was observed with either treatment.
Conclusions
TMZ MR 80 mg od and TMZ MR 35 mg bid have similar safety profiles. This new once-daily formulation could improve patient compliance with therapy, thereby enhancing clinical benefit.
Trial Registration
ISRCTN registry, ISRCTN 84362208.
Funding
Institut de Recherches Internationales Servier and Servier, Moscow, Russian Federation.
Plain Language Summary
Plain language summary available for this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain Language Summary
Angina is a condition affecting more than a 100 million patients worldwide. The drug trimetazidine has previously been shown to be efficacious for treating angina. Trimetazidine is currently available as 20- and 35-mg pills, which have to be taken two or three times daily, respectively. In the present study, a new formulation of 80 mg trimetazidine, for which one dose daily is sufficient, was compared to the existing 35 mg trimetazidine in terms of safety and was found to be similarly safe. Increased number of medications to be taken has previously been shown to decrease the probability of patients taking their treatment as prescribed. The fact that this new trimetazidine formulation reduces the number of pills taken daily could thus be a step towards helping patients to follow their treatment.
Introduction
The prevalence of angina pectoris (AP) is considerable, as it affects nearly 112 million people worldwide [1]. Despite available treatments, studies have reported that there is marked variability in achieving angina control [2, 3]. Although the extent of its impact is often underestimated by physicians [2, 4], angina can adversely affect patients’ quality of life and result in increased healthcare costs [5].
Beta-blockers, dihydropyridine calcium channel blockers, and nitrates, which are widely used as antianginal medications, affect cardiovascular hemodynamics, reducing oxygen demand and/or increasing oxygen supply. For patients remaining symptomatic despite monotherapy, European guidelines [6] recommend a combination of different antianginal agents. The addition of trimetazidine (TMZ) can provide an opportunity to optimize antianginal treatment, as it does not have any hemodynamic effect but acts directly at the myocardial cell level instead. By inhibiting an enzyme involved in fatty acid oxidation, TMZ leads to increased creatine phosphate/ATP (adenosine triphosphate) ratio and preservation of myocardial high-energy phosphate levels and ion pump function, ultimately resulting in improved cardiac efficiency [7]. The efficacy of TMZ in treating stable angina, both as monotherapy and in combination, has been reported and summarized in a meta-analysis of randomized clinical trials [8].
Non-adherence to treatment is frequently observed in outpatient care and can be observed even in symptomatic conditions like angina [9]. Decreasing the number of treatment doses taken per day could help to improve adherence [9,10,11] and thus potentially translate into clinical benefit. With this aim of simplification of the medication regimen, a once-daily (od) oral formulation of TMZ 80 mg was developed, with an equivalent systemic exposure to TMZ 35 mg twice daily (bid) demonstrated.
In the present 12-week study, the safety of TMZ modified-release (MR) 80 mg formulation was compared with the already-marketed TMZ MR 35-mg formulation, in patients with chronic stable angina.
Methods
The present study was an international, multicenter, randomized, double-blind, parallel-group phase III study with a treatment period of 12 weeks. The study was conducted in Russia and in Serbia, from January 2013 to August 2013. In total, 15 centers were selected and included at least one patient: 12 in Russia and three in Serbia.
Patients were both male and female, aged ≥ 21 years old, with chronic stable angina pectoris, where the symptoms were classified as being class 1, 2, or 3 according to the Canadian Cardiovascular Society (CCS) classification. Patient treatment had to include at least one regular antianginal medication such as beta-blockers, calcium channel blockers, long-acting nitrates, nicorandil, ivabradine, or molsidomine. Short-acting nitrates were administered on demand. At the time of selection, the patient had to be already treated by his/her physician for angina pectoris with TMZ MR 35 mg bid for at least 1 month, with satisfactory clinical effect and good tolerance. Coronary heart disease should have been documented by either previous MI (myocardial infarction) or previous coronary revascularization ≥ 3 months, or angiographic evidence of ≥ 50% narrowing of ≥ 1 major epicardial coronary artery, or in male patients only documented evidence of myocardial ischemia. The main exclusion criteria were current or previous Parkinsonian symptoms and abnormal renal function with estimated creatinine clearance (eCrCl) < 60 ml/min.
With respect to blinding, a double-dummy design was used, with placebo tablets matching TMZ MR 35-mg tablets and placebo capsules matching TMZ MR 80-mg capsules. The study consisted of a run-in period of 2 weeks, during which patients received TMZ MR 35 mg bid and one capsule of placebo twice daily, followed by a double-blind treatment period of 12 weeks, during which patients were randomized to receive either (A) one tablet of TMZ MR 35 mg and one capsule of placebo twice daily or (B) one capsule of TMZ MR 80 mg and one tablet of placebo in the morning and one capsule of placebo and one tablet of placebo in the evening. Randomization was not centralized. The treatments were allocated at the inclusion visit (week 0) by a balanced (non-adaptive) randomization (ratio 1:1) with stratification by center.
The main objective of the study was to assess safety of TMZ MR 80 mg od compared to TMZ MR 35 mg bid.
All of the 165 patients included in the study attended week 0 (W0), week 4 (W4), week 8 (W8), and week 12 (W12) visits for safety assessment. Safety evaluation was based on the following: (1) adverse events, (2) laboratory parameters (biochemical and hematological, only at inclusion/W0 and W12), (3) vital signs including weight (only at inclusion/W0 and W12), supine and standing blood pressure and pulse rate, (4) 12-lead electrocardiogram, and (5) CCS classification of symptoms of angina pectoris.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Statistical Analysis
Descriptive statistics were provided by treatment group (and all groups pooled for study outcome criteria and adverse events) depending on the nature of the criteria. For quantitative criteria, the number of observed values, mean, standard deviation, minimum and maximum, median, first and third quartiles were used. For qualitative criteria, the number of observed values, number, and percentage of patients per class were used.
Results
A total of 180 patients were screened and 177 were selected for the study. Among them, 165 patients (130 in Russia and 35 in Serbia) were included and randomly assigned to one of the two treatment groups: either the TMZ MR 80 mg od group (n = 82 patients) or the TMZ MR 35 mg bid group (n = 83 patients). Reasons for exclusion of selected patients were: non-compliance with inclusion/non-inclusion criteria in nine patients and adverse events in three patients. No patient was withdrawn from the study, nor lost to follow-up.
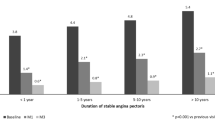
The main characteristics at baseline are summarized in Table 1. The two groups were well balanced regarding demographic characteristics at baseline (64 years old, 69% male, 100% Caucasian) and duration of stable angina pectoris (mean duration, 6.3 ± 5.4 years). In addition to angina pectoris, the most frequent medical histories related to CAD (coronary artery disease) were myocardial infarction (73%) and unstable angina (15%). Forty-two percent of the patients had previous coronary angioplasty and 26% previous coronary artery bypass. Before the treatment period, all of them were receiving specific treatment for angina, mainly beta-blocking agents (88%), calcium channel blockers (34%), and/or long-acting nitrates (32%), which was maintained during the study. Overall, 47.9% of the patients received additional concomitant treatment before the treatment period with no relevant difference between groups. They were mainly diuretics (20% of the patients) and drugs used in diabetes (18% of the patients). No major difference between the two groups was observed regarding blood pressure and heart rate, ECG (electrocardiogram), and laboratory parameters. There was a slightly higher proportion of patients with class I and class III CCS in the TMZ MR 80-mg group than in the TMZ MR 35-mg group. No major changes were reported regarding concomitant treatments during the study.
In the Safety Set (which consisted of all the randomized and treated patients, i.e., 165 patients), the proportion of patients who experienced at least one emergent adverse event (EAE) during the double-blind treatment period (Table 2) was slightly lower in the TMZ MR 80-mg group (14 patients, 17.1%) than in the TMZ MR 35-mg group (19 patients, 22.9%). In the TMZ MR 80-mg group, the most frequently reported EAE (at least two patients) was influenza (two patients), whereas in the TMZ MR 35-mg group it was hyperglycemia (four patients), hyperbilirubinemia (two patients), and atrial fibrillation (two patients). No EAE led to dose modification, withdrawal, or temporary interruption of the study treatments.
In terms of intensity, most of the EAEs were rated as mild (14 vs. 18 events, in the TMZ MR 80-mg and MR 35-mg group, respectively) or moderate (seven events in each group). Three patients in each group experienced serious adverse events (Table 3). None was considered to be related to treatment and all recovered.
In addition, only one event (polyuria, which recovered fully) in the TMZ MR 80-mg group was considered to be related to the study treatment by the investigator.
Most emergent adverse events recovered in both treatment groups: 75.0% of events in the TMZ MR 80-mg group and 56.0% in the TMZ MR 35-mg group. Events not recovered at the end of the study were mostly related to chronic diseases (i.e., diabetes, dyslipidemia) or were reported at the last visit (W12). None of them was serious or of severe intensity.
There was no study treatment interruption due to lack of efficacy. Regarding CCS classification of symptoms of angina, no worsening was reported with either treatment.
No clinically significant changes were observed during the study regarding vital signs (weight, blood pressure, and pulse rate), ECG, and laboratory parameters. Based on local laboratory tests, very few biological values reached a potentially clinically significant level (PCSA), each of them affecting only one patient, with the exception of high triglycerides in the TMZ MR 35-mg group, which affected three patients:
-
4 PCSA values in the TMZ MR 80-mg group: high GGT, low clearance creatinine, high triglycerides, and low platelets,
-
6 PCSA values in the TMZ MR 35-mg group: high glucose, low HDL cholesterol, high triglycerides, and high white blood cell count.
Discussion
This is the first study to assess the clinical acceptability of TMZ MR 80 mg, a new formulation of trimetazidine allowing a single dose daily. Previous pharmacokinetic studies performed with the 80-mg MR formulation (data on file) showed that:
-
This formulation presents plasma profiles of an MR once-daily formulation,
-
This formulation leads to AUC24, Cmax, and Cmin equivalent to the ones obtained with the MR 35 mg bid after repeated dosing in fed conditions in healthy volunteers,
-
There is no food effect on pharmacokinetic parameters.
In the present study, TMZ MR 80 mg once daily compared to TMZ MR 35 mg bid for a 12-week treatment period were found to have similar safety profiles in patients with documented coronary artery disease and stable chronic angina pectoris. Study treatment was given on top of both routine antianginal therapies and secondary prevention therapy. Patients were treated with TMZ MR 35 mg bid during the run-in period, therefore only a marginal additional benefit was expected on angina symptoms during the double-blind treatment period. According to the summary of product characteristics and minimization measure implemented to reduce the risk of overexposure to TMZ in patients with renal insufficiency, patients with moderate-to-severe renal impairment (creatinine clearance below 60 ml/min during the run-in period) were excluded, since no adaptation of the dose could be done with the capsules containing 80 mg of TMZ.
TMZ is a well-established therapy for angina control, with a mechanism of action that is distinct from that of other antianginal agents, as it acts directly at the level of myocardial cells. Since it is devoid of any major hemodynamic properties, it can be combined with other classes of antianginal therapy. The MR 80-mg formulation taken od simplifies the treatment regimen and would thereby be expected to improve adherence to treatment in real-life settings.
Non-adherence to treatment is known to be a problem in asymptomatic diseases, with the notable example of hypertension, but can also concern symptomatic diseases, and has been reported in patients with stable angina treated with symptom-releasing nitrates [9]. Amongst patients with angina, adherence could be hampered by the total number of medications prescribed, since many of the patients have multiple comorbidities, such as diabetes mellitus or hypertension. Adherence has been shown to be related not only to the number of medications but also to the number of doses to be taken per day [12] and it is inversely related to the latter [13]. Hence, simplifying a medication regimen might be expected to be associated with improved adherence and so with potential increased clinical benefits in patients with angina. A study showed that converting patients with chronic, stable angina to long-acting antianginal medications led to substantial improvement in symptom control [10]. Improved adherence with once-daily treatment compared with twice-daily treatment was shown for both beta-blocker and nitrate regimen [9, 11]. Health-related quality of life was also improved according to one study [11].
In light of this evidence, the availability of a once-daily formulation of TMZ, with similar safety to the twice-daily formulation, could offer an opportunity for improving patient adherence to treatment.
Study Limitations
Despite its randomized, double-blind design, the study has limitations related to the small size of the patient population (n = 165 patients) and to the protocol selection criteria.
Conclusions
TMZ MR 80 mg od was shown to have a similar safety profile to TMZ MR 35 mg bid. This once-daily formulation could possibly help increase treatment adherence and thus lead to clinical benefit in real life.
References
Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96.
Beltrame JF, Weekes AJ, Morgan C, Tavella R, Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: the Coronary Artery Disease in General Practice (CADENCE) Study. Arch Intern Med. 2009;169:1491–9.
Kureshi F, Shafiq A, Arnold SV, et al. The prevalence and management of angina among patients with chronic coronary artery disease across US outpatient cardiology practices: insights from the Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) study. Clin Cardiol. 2017;40:6–10.
Qintar M, Spertus JA, Gosch KL, et al. Effect of angina under-recognition on treatment in outpatients with stable ischaemic heart disease. Eur Heart J Qual Care Clin Outcomes. 2016;2:208–14.
Arnold SV, Morrow DA, Lei Y, et al. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes. 2009;2:344–53.
Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003.
Fragasso G, Perseghin G, De Cobelli F, et al. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006;27:942–8.
Danchin N, Marzilli M, Parkhomenko A, Ribeiro JP. Efficacy comparison of trimetazidine with therapeutic alternatives in stable angina pectoris: a network meta-analysis. Cardiology. 2011;120:59–72.
Kardas P, On behalf of COMPASS investigators. Comparison of once daily versus twice daily oral nitrates in stable angina pectoris. Am J Cardiol. 2004;94:213–6.
Spertus JA, Dewhurst T, Dougherty CM, Nichol P. Testing the effectiveness of converting patients to long-acting antianginal medications: the Quality of Life in Angina Research Trial (QUART). Am Heart J. 2001;141:550–8.
Kardas P. Compliance, clinical outcome, and quality of life of patients with stable angina pectoris receiving once-daily betaxolol versus twice daily metoprolol: a randomized controlled trial. Vasc Health Risk Manag. 2007;3:235–42.
Cramer JA. Effect of partial compliance on cardiovascular medication effectiveness. Heart. 2002;88:203–6.
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–310.
Acknowledgements
The author would like to thank the investigators of the study.
Arkhangelsk: Uskov V.L. Barnaul: Gon-charenko I.I., Prasolova T.P. Belgorod: Guseva V.G., Shinkar A.S. Bryansk: Samsonova S.M. Veliky Novgorod: Vikhrova I.V., Kuz’kina S.A. Vladimir: Mitina L.V., Timofeeva I.V. Vol-gograd: Archakova T.M., Kovaleva N.Y., Romanova E.A., Tivon Y.V. Vologda: Antonova Y.N., Kurganova O.B. Voronezh: Davydova N.N., Klyuchantseva O.V., Popovskaya Y.V., Kharitonova E.I. Evpatoria: Kuzmina T.N. Ekaterinburg: Buzmakova K.V., Kaplenko L.I., Pospelova N.V., Stepanova A.Y. Ivanovo: Kol-basheva N.A. Irkutsk: Krasnova G.M., Pal’vin-skaya A.Y., Toloknova V.A. Kazan: Bikmullina R.F., Gainullina A.A., Kedrina E.V., Mikhailova S.A., Nabiullina T.A., Nizamova A.F. Kaluga: Uskova A.A., Yushkova A.E. Kemerovo: Andreeva O.V., Fedotova G.V. Kirov: Besser-geneva O.L., Gavrilyuk D.D., Ehalo N.V., Zlo-bina M.V. Krasnodar: Zhemartseva E.Y., Markushina I.A., Pavlovets V.P., Sobolenko A.A. Krasnoyarsk: Apanovich I.E., Kireeva N.V., Maksimova I.V. Kursk: Butz T.V., Pavlova I.A. Lipetsk: Bachurina S.N., Orlyachenko S.V. LR Sertolovo: Zaitseva T.V., LR Lomonosov: Beznogova V.F. LR-South: Litsis N.N., Novozhenina A.Y. Moscow: Abramyan L.L., Adamyan M.M., Askerko S.N., Bolmosov A.N., Vasilieva I.N., Volodova S.I., Grishko P.V., Zherebetskaya E.S., Zemlyanaya N.S., Klysh-nikova L.N., Kononchik E.I., Kuznetsova N.A., Kuz’minova I.A., Marmurova I.V., Mikhailova R.Y., Mordovina I.P., Nazarkina O.V., Per-epechko A.P., Pivovarova N.G., Potapova T.P., Prokofiev D.A., Proniushkina N.E., Savelieva E.V., Semovskikh N.A, Timonenkova L.D., Fomin V.V., Furman O.A., Tsutsieva R.M., Chibrikina M.V., Shoshina I.N., Yashchenko.P. Moscow region: Bocharova T.I., Demya-nenko O.L., Zhukova L.B., Melnikov A.Y., Mer-kulieva I.A., Tyasina E.I., Pakholkova N.S., Rogozina S.V., Chugunova I.V. Murmansk: Brazhnik M.L., Guseva Y.V. Naberezhnye Chelny: Anisimova A.N., Kuzeyina S.S., Kulakhmetova R.G., Petrova I.S. Nizhny Nov-gorod: Ignatyeva I.A., Morozova T.A., Ryb-nikova N.V., Gritsenko I.I. Novokuznetsk: Kondratskaya O.V., Shishkin A.V. Novosibirsk: Gogleva N.N., Kulipanova V.M., Mitrofanova S.V., Parada E.V., Svistunova S.Y., Sergeeva T.M. Omsk: Kryukova V.V., Suprun T.N., Fedorova E.M., Shnor O.F. Orel: Mitroshina T.N., Shemetova T.S., Orenburg: Val’kevich L.P., Varnikova S.N. Penza: Ivanova E.A. Perm: Shlykova O.N. Pyatigorsk: Guryanova I.R., Zheltova V.L. Rostov-on-Don: Bulygina E.D., Gorskaya E.V., Kosenko L.V., Musaeva F.K., Fedorchenko M.Y., Harish V.I. Ryazan Region: Serbarinova O.M. Ryazan: Yatsenya Y.A. Sa-mara: Golubev M.N., Kopaev D.E., Miludina L.A., Polischuk L.V., Shilintseva O.A., Krylova, L.M. St. Petersburg: Vasilik M.V., Zotov D.D., Kalishevich N.B., Kachmazova L.I., Kontorikova S.G., Mamoshko T.A., Osnovin S.A., Timosh-enko (Schmalz) I.O., Kashina A.N., Kiryanova O.G. Saratov: Kotova L.E., Kuvshinova L.E., Ulyanova I.M., Shevelo O.F. Simferopol: Kir-eeva I.B., Korohova L.V., Smolensk: Lisunova T.I., Medvedeva E.V. Stavropol: Matvienko T.E., Shovgaryan S.L. Stary Oskol: Nebolsina T.F. Syktyvkar: Mikusheva M.A., Misharin N.N. Tver Region: Kutaliya T.O. Tolyatti: Chernova V.N., Yanina Y.A. Tomsk: Permyakova O.V., Skurikhina N.N. Tula: Goldinova L.M., Pri-khodko T.N. Tyumen: Myshyakova A.G. Ufa: Akhmerova E.Z., Zaitseva K.V., Ozerchuk A.A., Polyakova I.M., Rodionova, Safiullina I.D. Cheboksary: Arsentieva I.N., Volkova O.O. Chelyabinsk: Kondrina I.N., Kharlova T.E. Yalta: Grigorieva T.L. Yaroslavl: Kurtmulaeva K.V., Rogozina O.M.
Funding
Sponsorship for this study was provided by the Institut de Recherches Internationales Servier and Servier, Moscow, Russian Federation. Article processing charges for this study were funded by Servier. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing
Writing assistance was provided by Diana Toli, PhD (Servier). Writing assistance was funded by Servier.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval for the version to be published.
Authorship Contributions
The author would like to thank all participating investigators for their contribution to the study.
Prior Presentation
The data presented in this manuscript were previously presented in 2016 at the 38th Congress of the European Society of Cardiology (ESC) as a poster entitled: Clinical acceptability of trimetazidine 80 mg once daily versus trimetazidine modified-release 35 mg twice daily in chronic stable angina pectoris. Eur Heart J 2016 Vol. 37 (suppl. 1): 388 (P1857). Y. Pozdnyakov. Moscow Regional Cardiology Center, Cardiology, Zhukovsky, Russian Federation.
Disclosures
Yuri M. Pozdnyakov, scientific coordinator of this study, received grants and honoraria for conducting research and honoraria for lectures from Servier, Moscow, Russian Federation.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The members of the study investigators are listed in acknowledgements.
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6165716.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pozdnyakov, Y.M., On behalf of the study investigators. Clinical Acceptability Of Trimetazidine Modified-Release 80 mg Once Daily Versus Trimetazidine Modified-Release 35 mg Twice Daily In Stable Angina Pectoris. Cardiol Ther 7, 61–70 (2018). https://doi.org/10.1007/s40119-018-0110-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-018-0110-5