Abstract
Introduction
Trimetazidine (TMZ) has been shown to be efficacious for angina treatment. The TMZ 80-mg formulation allows one-daily (od) dosage, which could improve symptom control and adherence.
Methods
The 3-month, observational, multicenter, prospective ODA (antianginal effectiveness and tolerability of trimetazidine modified release 80 mg Once Daily in stable Angina patients in real-world practice) study assessed TMZ 80 mg od effectiveness in stable angina patients with persistent symptoms despite therapy. Two clinical situations were compared: patients who initiated treatment with TMZ 80 mg od (initiation group) and patients who were previously treated with TMZ 20 mg thrice daily (tid) or TMZ 35 mg MR twice daily (bid) and switched to TMZ 80 mg od (switch group). Number of angina attacks, short-acting nitrate (SAN) consumption, self-reported patient daily activity, Canadian Cardiovascular Society (CCS) class, adherence to antianginal therapy, overall efficacy and tolerability were assessed.
Results
A significant decrease in weekly number of angina attacks was observed for both the initiation group (n = 1841 patients) from 4.8 ± 3.5 at baseline to 0.9 ± 1.4 at 3 months (M3) (P < 0.001), and the switch group (n = 1216 patients) from 4.4 ± 1.3 at baseline to 0.9 ± 1.3 at M3 (P < 0.001). Significant reduction in SAN consumption and improvement in CCS class were observed for both groups. Adherence to antianginal therapy improved in both groups at 1 month (M1) and M3. Overall effectiveness of TMZ 80 mg od was rated by physicians as “very good” (68% initiation group, 70% switch group), “good” (31% initiation group, 29% switch group), “moderate” (1%, both groups) or “poor” (< 1%, both groups). Overall tolerability of TMZ 80 mg od was rated by physicians as “very good” (75%), “good” (25%) or “moderate” (< 1%) in both groups.
Conclusions
TMZ 80 mg od, in association with other antianginal therapy, effectively reduced angina attacks and SAN consumption and improved physical activity and adherence to antianginal therapy both in patients initiating TMZ treatment and those switching from a bid or tid formulation.
Trial Registration
ISRCTN registry Identifier, ISRCTN97780949.
Funding
Servier.
Plain Language Summary
Plain language summary available for this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain Language Summary
Angina is a condition that affects more than 100 million patients worldwide. The drug trimetazidine has been shown to be efficacious for angina treatment. In the present study, two groups of patients were analyzed. One group started treatment with a formulation of trimetazidine that allows patients to take only one pill per day. The other group was previously treated with other trimetazidine formulations that had to be taken two or three times a day, and then switched to the once-daily treatment. In both cases, treatment with the new formulation decreased the number of angina attacks and the use of nitroglycerine. It also improved physical activity as reported by patients. Moreover, the number of patients taking their antianginal medicine as prescribed was increased. This new formulation could provide an opportunity to improve angina symptoms and increase the number of patients following their treatment.
Introduction
Angina pectoris is a common manifestation of coronary artery disease and can have a considerable impact on a patient’s quality of life [1], but despite currently available therapies, it is not always satisfactorily managed [2,3,4].
Trimetazidine (TMZ) differs from other antianginal agents in that it acts directly at the myocardial cell level [5]. Add-on TMZ thus represents an opportunity to optimize antianginal treatment. Its efficacy in stable angina treatment, as monotherapy and in combination, has been reported in a meta-analysis of randomized clinical trials [6].
TMZ is now available as a new formulation of 80 mg allowing once-daily (od) intake. The ODA (Anti-anginal effectiveness and tolerability of trimetazidine modified release 80 mg Once Daily in stable Angina patients in real-world practice) study [7] assessed in a real-life setting the effectiveness and tolerability of TMZ 80 mg od, as well as patient adherence to antianginal treatment, in chronic stable angina patients with persistent symptoms despite therapy. Herein, we report an additional analysis of the ODA study, in patients initiating treatment with TMZ 80 mg od and in patients switching to TMZ 80 mg od from previous treatment with TMZ 20 mg tid (thrice daily) or TMZ 35 mg MR bid (twice daily).
Methods
ODA was a 3-month observational, multicenter, prospective study in 3066 stable angina patients with persistent symptoms despite therapy, conducted in Russia from March 2017 to June 2017 in a real-world clinical setting. The methods and main findings of this study have been previously reported [7].
Patients were treated in line with current recommendations for coronary artery disease management. Inclusion of patients into the study was exclusively determined by the decision of the physician regarding medical meaningfulness and indication for treatment with TMZ od 80 mg. Non-inclusion criteria were detailed previously [7].
For the analyses presented herein, patients for whom information on previous treatment with TMZ was available (n = 3057) were divided into two groups: (i) one group of patients who had not been previously treated with TMZ and initiated treatment with TMZ 80 mg od (initiation group) and (ii) one group of patients who were previously receiving treatment with either TMZ 20 mg tid or TMZ 35 mg MR bid and then switched to treatment with TMZ 80 mg od (switch group). Of the 3066 patients of the ODA study population, nine patients were excluded from this analysis, because it was not possible to classify them into one of the two groups (initiation or switch), due to lack of information on previous use of TMZ.
Data were collected at three visits: at baseline, at 1 month (M1) and at 3 months (M3). At each of the study visits, data were collected on the number of angina attacks, consumption of short-acting nitrates (SAN) within the week prior to the visit, evaluation of Canadian Cardiovascular Society (CCS) classification, patient self-assessment of their daily physical activity, and adherence. At the last study visit, a general assessment of tolerability and effectiveness of TMZ 80 mg od therapy was provided by physicians (rated as ‘very good’, ‘good’, ‘moderate’ or ‘poor’).
For self-assessment of physical activity, patients were asked to rate how angina impacted their daily activity on a scale of 1 to 10 (1—no limitations, 10—very marked reduction). Answers were categorized into five categories: no limitation (0), slight limitation (1–2), moderate limitation (3–4), substantial limitation (5–7), and very marked reduction (8–10).
Adherence to antianginal therapy was assessed by using a previously published six-item questionnaire [8], with the following definitions: good adherence—patient responded “NO” to all questions; moderate adherence—patient responded “YES” to 1–2 questions; non-adherence—patient responded “YES” to three or more questions. As a part of the assessment of adherence to TMZ, patients were asked to rate how satisfied they were with TMZ therapy, on a scale of 1—not satisfied, to 10—very satisfied, at M1 and M3.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This study was approved by the Interuniversity Ethical Committee, Moscow.
Statistical Analysis
A descriptive statistical analysis was performed, assisted by SAS software, version 9.1. Patients were analyzed if they had valid data from all visits. All parameters were analyzed using descriptive statistics methods. The number of patients, mean value, standard deviation, minimum and maximum value or proportion by category were specified for each parameter. Differences in the numbers of angina pectoris episodes and in the necessity to use antianginal drugs were evaluated by the Wilcoxon’s signed-rank test. A p < 0.05 was considered to be significant. The dynamics of the parameters analyzed from visit to visit (i.e., for the blood pressure, the efficacy of therapy etc.) were studied using both Wilcoxon signed-rank test and Student’s paired t test.
Results
In the present analysis, the ODA study [7] population was divided into two groups: the initiation group, comprising 1841 patients who initiated treatment with TMZ 80 mg od, and the switch group, comprising 1216 patients previously treated with TMZ 20 mg tid (n = 84 patients) or TMZ 35 mg MR bid (n = 1132 patients) and who switched to treatment with TMZ 80 mg od.
Demographic and baseline characteristics are summarized in Table 1. The proportion of patients with class II angina was higher in the initiation group (57.6 vs. 52.3% in the switch group), while the proportion of those with class III angina was higher in the switch group (30.0 vs. 26.0% in the initiation group). With regard to medical history, a higher proportion of patients in the switch group had previous MI (32.8 vs. 27.1% in the initiation group), percutaneous coronary intervention/coronary artery bypass grafting (39.1 vs. 17.1% in the initiation group), and diabetes mellitus (22.0 vs. 19.7% in the initiation group). Patients in the initiation group had a higher average baseline SBP (142.0 ± 16.2 mmHg vs. 139.9 ± 15.7 mmHg in the switch group). Baseline medications were similar in both groups, with the exception of statins (65.8% of patients in the initiation group vs. 74.0% in the switch group).
A significant improvement in CCS class was observed in both treatment groups at M1, and a further improvement was observed at M3 (Table 2).
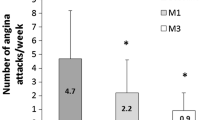
Treatment with TMZ 80 mg od, whether in the initiation or the switch group, led to a significant decrease in weekly angina attack frequency from 4.8 ± 3.5 at baseline to 2.3 ± 2.5 at M1 (P < 0.001) and to 0.9 ± 1.4 at M3 (P < 0.001) in the initiation group (Fig. 1a), and from 4.4 ± 1.3 at baseline to 2.2 ± 2.5 at M1 (P < 0.001) and to 0.9 ± 1.3 at M3 (P < 0.001) in the switch group (Fig. 1b), without intergroup difference.
The average consumption of SAN per week decreased from 4.5 ± 3.9 at baseline to 2.0 ± 2.6 at M1 (P < 0.001) and to 0.7 ± 1.3 at M3 (P < 0.001) in the initiation group (Fig. 1a), and from 4.2 ± 3.7 at baseline to 1.9 ± 2.4 at M1 (P < 0.001) and to 0.7 ± 1.3 at M3 (P < 0.001) in the switch group (Fig. 1b), without intergroup difference.
Physical activity, as self-assessed by patients, was improved in both groups (Fig. 2), as evidenced by a progressive increase from baseline to M1 and then to M3 in the proportion of patients who reported no limitation or slight limitation and by a progressive decrease in the proportion of patients who reported substantial limitation or very marked reduction.
Adherence to antianginal treatment improved significantly in both groups (Table 3). In the initiation group, good adherence was reported by 54% of patients at M3 vs. 25% at baseline (p < 0.001) and vs. 38% at M1 (p < 0.001). In the switch group, good adherence was reported by 59% of patients at M3 vs. 21% at baseline (p < 0.001) and vs. 38% at M1 (p < 0.001). Non-adherence decreased from 34% at baseline to 8% at M1 (p < 0.001) and further decreased to 3% at M3 (p < 0.001 vs. baseline) in the initiation group. Similarly, in the switch group, the proportion of non-adherent patients decreased from 38% to 8% at M1 (p < 0.001) and to 3% at M3 (p < 0.001 vs baseline).
At the global assessment performed at the last visit, overall effectiveness of TMZ 80 mg od was rated by physicians as “very good” (67.8% in the initiation group vs. 69.8% in the switch group), “good” (30.7% in the initiation group vs 29.5% in the switch group), “moderate” (1.1% in the initiation group vs 0.7% in the switch group) or “poor” (0.3% in the initiation group vs 0.1% in the switch group). Overall tolerability was rated by physicians as “very good” (75.2% in both groups), “good” (24.7% in the initiation group vs. 24.8% in the switch group) or “moderate” (0.1% in the initiation group and 0% in the switch group).
Discussion
In the present report, we examined the effect of the addition of TMZ 80 mg od in two clinical situations: patients initiating treatment with TMZ 80 mg od and patients switching to TMZ 80 mg od from previous treatment with TMZ 20 mg tid or TMZ 35 mg MR bid. In both groups, we observed a significant decrease in angina attack frequency and in SAN consumption, as well as an improvement in CCS classification and in self-reported patient physical activity. These results are in line with the findings reported for the overall population of the ODA study [7]. Of note, the proportion of patients with CCS Class I increased by four times by M3 in both groups. Achievement of CCS Class I is very important for quality of life, which is one of the main objectives of antianginal treatment according to guidelines [9].
TMZ 80 mg od has been shown in a randomized double-blind phase III study to have similar safety profile to TMZ MR 35 mg bid [10] and was well tolerated in a real-world setting [7].
The mechanism of action of TMZ differs from that of other antianginal drugs. It targets directly myocardial cells, optimizing cellular energetics particularly under ischemic conditions [5], which makes it a valuable antianginal therapy regardless of the background medications [11]. The antianginal efficacy of TMZ has been shown in controlled trials in patients with stable angina treated with TMZ in monotherapy or as a part of combination therapy [12,13,14,15,16]. In monotherapy, the antianginal efficacy of TMZ was shown versus placebo [12]. TMZ was shown to have similar efficacy to other classes of antianginal drugs, such as diltiazem or propranolol [13, 14]. In a meta-analysis that included 218 trials with a total of 19,028 patients, TMZ significantly improved exercise tolerance, weekly angina episodes and use of SAN compared with placebo. TMZ efficacy was comparable to that of other non-heart-rate-lowering antianginal treatments [6]. It has also been shown that increasing the number of hemodynamic drugs does not increase antianginal efficacy [15,16,17,18]. On the contrary, combining TMZ with hemodynamic agents (beta-blockers or long-acting nitrates) significantly improved exercise stress test parameters and angina symptoms, as demonstrated in the TACT study [19]. The TRIMPOL study also showed that treatment with metoprolol and trimetazidine led to significant improvement in exercise stress tests and angina symptoms compared to metoprolol and placebo [20]. Finally, in patients with stable effort-induced angina not sufficiently controlled with propranolol, the addition of trimetazidine led to better antianginal efficacy than addition of isosorbide dinitrate [21]. All these data provide evidence that TMZ is an efficacious therapy to improve angina control. The results of the present study provide additional evidence of the effectiveness of TMZ in a real-life setting, as initiation of TMZ 80 mg od resulted in significant decrease in angina attacks and SAN consumption and improved functional status as assessed by CCS classification and physical activity.
Moreover, adherence to antianginal treatment was improved in the present study. The improvement in adherence observed when patients switched from TMZ bid or tid formulation to TMZ od formulation could be explained by treatment simplification, as adherence is inversely related to the number of medication doses to be taken per day [22]. However, as adherence was also improved in the initiation group, the increase in adherence could be related to the reduction in angina symptoms and the improvement in daily activity as perceived by patients. Moreover, we cannot exclude that participation to the trial could have had a beneficial effect on adherence.
The clinical implications of these findings are that TMZ addition provides a useful therapeutic strategy for clinicians, with regard to several aspects: antianginal effectiveness, adherence improvement, and patient-reported improvement with regard to their daily physical activity.
Study Limitations
The study has limitations inherent in the nature of its design (open-label, observational), which may have resulted in bias toward overestimating the treatment effect. Another limitation is the lack of a control group, which might have biased the results by overestimating the treatment effect. The short duration of follow-up (3 months) in this chronic condition is another limitation. Further investigations should be undertaken to confirm these results over a longer period of time. The tools used to test physical activity were not previously validated. Results are based on patient history and self-evaluation of angina and functional status, which can be a limitation. However, this allowed assessing the main objective of antianginal treatment, which is the reduction of symptoms and the improvement of quality of life.
Conclusions
In the present analysis of a prospective observational study over a 3-month period in daily clinical practice, TMZ 80 mg od, in association with other antianginal therapy, effectively reduced angina attacks and nitrate consumption and improved daily physical activity, CCS class and self-reported adherence to antianginal treatment both in patients initiating TMZ treatment and in those switching from a bid or tid formulation.
References
Moran AE, Forouzanfar MH, Roth GA, et al. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–501.
Beltrame JF, Weekes AJ, Morgan C, Tavella R, Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: the Coronary Artery Disease in General Practice (CADENCE) Study. Arch Intern Med. 2009;169:1491–9.
Kureshi F, Shafiq A, Arnold SV, et al. The prevalence and management of angina among patients with chronic coronary artery disease across US outpatient cardiology practices: insights from the Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) study. Clin Cardiol. 2017;40:6–10.
Qintar M, Spertus JA, Gosch KL, et al. Effect of angina under-recognition on treatment in outpatients with stable ischaemic heart disease. Eur Heart J Qual Care Clin Outcomes. 2016;2:208–14.
Fragasso G, Perseghin G, De Cobelli F, et al. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006;27:942–8.
Danchin N, Marzilli M, Parkhomenko A, Ribeiro JP. Efficacy comparison of trimetazidine with therapeutic alternatives in stable angina pectoris: a network meta-analysis. Cardiology. 2011;120:59–72.
Glezer MG, Vygodin VA; ODA investigators. Anti-anginal effectiveness and tolerability of trimetazidine modified release 80 mg once daily in stable angina patients in real-world practice. Adv Ther. 2018; 35:1368–1377.
Girerd X, Radauceanu A, Achard JM, et al. Evaluation of patient compliance among hypertensive patients treated by specialists. Arch Mal Coeur Vaiss. 2001; 94: 839–42 (Article in French).
Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003.
Pozdnyakov YM; study investigators. Clinical acceptability of trimetazidine modified-release 80 mg once daily versus trimetazidine modified-release 35 mg twice daily in stable angina pectoris. Cardiol Ther. 2018;7:61–70.
Glezer M; CHOICE-2 study investigators. The effectiveness of trimetazidine treatment in patients with stable angina pectoris of various durations: results from the CHOICE-2 Study. Adv Ther 2018; 35:1103–1113.
Passeron J. Effectiveness of trimetazidine in stable effort angina due to chronic coronary insufficiency. A double-blind versus placebo study. Presse Med. 1986;15:1775–1778.
Koylan N, Bilge AK, Adalet K, Mercanoglu F, Buyukozturk K. Comparison of the effects of trimetazidine and diltiazem on exercise performance in patients with coronary heart disease. The Turkish trimetazidine study (TTS). Acta Cardiol. 2004;59: 644–650.
Detry JM, Sellier P, Pennaforte S, Cokkinos D, Dargie H, Mathes P. Trimetazidine: a new concept in the treatment of angina. Comparison with propranolol in patients with stable angina. Trimetazidine European Multicenter Study Group. Br J Clin Pharmacol. 1994;37:279–288.
Fox KM, Mulcahy D, Findlay I, Ford I, Dargie HJ. The Total Ischaemic Burden European Trial (TIBET). Effects of atenolol, nifedipine SR and their combination on the exercise test and the total ischaemic burden in 608 patients with stable angina. The TIBET Study Group. Eur Heart J. 1996; 17:96-103.
Pehrsson SK, Ringqvist I, Ekdahl S, Karlson BW, Ulvenstam G, Persson S. Monotherapy with amlodipine or atenolol versus their combination in stable angina pectoris. Clin Cardiol. 2000;23:763–70.
Madjlessi-Simon T, Fillette F, Mary-Krause M, Lechat P, Jaillon P. Effects of amlodipine on transient myocardial ischaemia in patients with a severe coronary condition treated with a beta-blocker. Amlor-Holter Study Investigators. Eur Heart J. 1995;16:1780–8.
Savonitto S, Ardissiono D, Egstrup K, Rasmussen K, Bae EA, Omland T, Schjelderup-Mathiesen PM, Marraccini P, Wahlqvist I, Merlini PA, Rehnqvist N. Combination therapy with metoprolol and nifedipine versus monotherapy in patients with stable angina pectoris. Results of the International Multicenter Angina Exercise (IMAGE) Study. J Am Coll Cardiol. 1996;27:311-6.
Chazov EI, Lepakchin VK, Zharova EA, et al. Trimetazidine in Angina Combination Therapy—the TACT study: trimetazidine versus conventional treatment in patients with stable angina pectoris in a randomized, placebo-controlled, multicenter study. Am J Ther. 2005;12:35–42.
Szwed H, Sadowski Z, Elikowski W, et al. Combination treatment in stable effort angina using trimetazidine and metoprolol: results of a randomized, double-blind, multicentre study (TRIMPOL II). TRIMetazidine in POLand. Eur Heart J. 2001;22:2267–74.
Michaelides A, Spiropoulos K, Dimopoulos K, Athanasiades D, Toutouzas P. Antianginal efficacy of the combination of trimetazidine-propranolol compared with isosorbide dinitrate-propranolol in patients with stable angina. Clin Drug Invest. 1997;13:8–14.
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–310.
Acknowledgements
The authors would like to thank the participants of the study.
Funding
Sponsorship for this study was provided by Servier, Moscow, Russian Federation. Editorial assistance and article processing charges were funded by Servier, France. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Writing and editorial assistance was provided by Dr. Diana Toli (Servier) and funded by Servier, France.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
List of Investigators
The full list of ODA study investigators is available in the supplementary material.
Prior Presentation
The data presented in this manuscript have been previously presented as a poster at the congress of the European Society of Cardiology (ESC) in 2018.
Disclosures
Maria G. Glezer, scientific coordinator of this study, received honoraria for lectures from Servier, Moscow, Russian Federation. Vladimir A. Vygodin has nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This study was approved by the Interuniversity Ethical Committee, Moscow.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The full list of ODA study investigators is available in the supplementary material.
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7628735.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Glezer, M.G., Vygodin, V.A. & on behalf of ODA investigators. Effectiveness of Long-acting Trimetazidine in Different Clinical Situations in Patients with Stable Angina Pectoris: Findings from ODA Trial. Cardiol Ther 8, 69–78 (2019). https://doi.org/10.1007/s40119-019-0128-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-019-0128-3