Abstract
Introduction
Modus Vivendi was conducted in routine clinical practice to evaluate the effect of adding trimetazidine 80 mg once daily (TMZ 80 OD) to treat patients with persistent symptoms despite treatment with background antianginal therapies including maximally tolerated bisoprolol.
Methods
This multicenter, prospective, observational, open-label, uncontrolled study recruited adult outpatients with a confirmed diagnosis of stable angina to whom physicians had decided to prescribe TMZ 80 OD. All patients were symptomatic despite treatment, including maximally tolerated doses of bisoprolol. Data on number of angina attacks, use of short-acting nitrates, and quality of life (QoL) were collected at baseline (V1) and at 1-month (V2) and 3-month (V2) follow-up visits. Two sub-analyses assessed efficacy in patients who remained on a stable bisoprolol dose throughout the study, and in patients in whom background antianginal therapy was known.
Results
A total of 1939 patients were recruited (57.2% women). The mean age was 65.6 ± 8.8 years; 73.8% had class II and 26.2% class III angina. At V1, the mean number of angina attacks per week was 6.2 ± 6.5 despite antianginal therapy including maximally tolerated bisoprolol dosage. Following the addition of TMZ 80 OD, this decreased to 3.4 ± 4.2 attacks per week at V2, and 1.6 ± 2.6 at V3 (P < 0.05 at V2 and V3), with concomitant reductions in short-acting nitrate use (P < 0.05). Significant improvements in QoL were observed throughout the study. Subgroup analyses showed that the addition of TMZ 80 OD to guideline-recommended antianginal therapy was associated with significant reductions in the mean number of weekly angina attacks and consumption of short-acting nitrates and improvements in QoL whether patients were treated with maximally tolerated bisoprolol and TMZ 80 OD alone, or maximally tolerated bisoprolol and TMZ 80 OD on top of other antianginal therapies. Treatment was well tolerated.
Conclusion
The study findings support the addition of TMZ 80 OD to bisoprolol with or without other antianginal therapies for patients with persistent angina.
Trial Registration
This study was retrospectively registered under the number ISRCTN29992579.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Despite a range of pharmacotherapy options, many patients continue to suffer from anginal symptoms. TMZ has a metabolic mechanism of action and can reduce symptoms of angina in patients resistant to hemodynamic antianginal agents. |
This study assessed the efficacy and safety of a once-daily formulation (TMZ 80 OD) in patients with persistent symptoms despite treatment with guideline-recommended antianginal therapies including maximally tolerated bisoprolol. |
What was learned from the study? |
The addition of TMZ 80 OD to other antianginal therapies including maximally tolerated bisoprolol was associated with significant decreases in mean weekly angina attacks and consumption of short-acting nitrates, along with improvements in quality of life, independent of background therapy. |
TMZ 80 OD can be safely combined with first-line antianginal therapies in patients with persistent angina. |
Introduction
Angina pectoris is estimated to affect around 71 million people worldwide [1] and is often the first warning sign of an underlying chronic coronary syndrome (CCS). Data from a recent survey of CCS patients treated in primary care reported that 21.2% experienced angina at least monthly, with 12.5% reporting daily or weekly angina [2].
Persistent angina symptoms have a major negative impact on health-related quality of life (QoL) including poor general health status, anxiety, depression, and inability to self-manage [3]. They also make a greater contribution to years lived with disability than either myocardial infarction or heart failure [1, 4].
In addition to the personal consequences of an angina diagnosis, there is also an associated economic burden [5, 6]. This is related to direct costs in the form of hospitalizations, surgery, and medications, as well as indirect costs in the form of angina-related work limitations or unemployment. There is therefore significant rationale for patients with stable angina to be diagnosed early and managed appropriately.
The treatment of stable angina has two major goals: first, to prevent more serious cardiovascular events such as myocardial infarction or death, and second, to improve patients' QoL by alleviating symptoms caused by ischemia [7]. Although randomized controlled trials show that antianginal drugs are equally effective in terms of symptom relief [8], guidelines continue to recommend the use of beta-blockers and calcium channel blockers (CCB) as first-line treatments in combination with effective risk factor modification and lifestyle changes [7]. Long-acting nitrates represent the first second-line addition, and newer second-line drugs such as nicorandil, ranolazine, ivabradine, and trimetazidine have class IIa recommendations when symptoms are not adequately controlled by first-line drugs and nitrates [7]. As beta-blocker positioning has remained essentially unchanged in successive guidelines, they remain one of the most widely used antianginal agents in clinical practice [9, 10]. Data from large international registries have shown that two-thirds (REACH) to three-quarters (CLARIFY) of patients with stable CCS receive beta-blockers alone or in combination [11, 12]. Among the beta-blockers, bisoprolol is the most frequently prescribed agent [11]. Nevertheless, a number of large studies have shown that angina remains a therapeutic problem despite treatment with conventional first-line hemodynamic antianginal therapies [13,14,15]. A significant number of patients may therefore benefit from treatment with an agent with a different mechanism of action from those associated with traditional antianginal therapy.
Current first-line agents improve myocardial oxygen supply or reduce demand via a range of hemodynamic actions [16]. Beta-blockers and calcium channel blockers decrease heart rate and cardiac cell contractility, resulting in a decrease in myocardial oxygen demand. Calcium channel blockers also cause coronary artery vasodilation, which can increase oxygen supply.
Trimetazidine (TMZ) is an antianginal drug devoid of hemodynamic effect, which optimizes energy metabolism in the ischemic heart by acting directly at the cellular level to inhibit free fatty acid oxidation and thereby shift cardiac cell metabolism to glucose oxidation. In this manner, the reduced oxygen supply to the heart is optimized and the integrity and function of the cardiac cells is preserved during the ischemic episode [17]. As a result of this mechanism of action, TMZ can reduce symptoms of angina in patients resistant to hemodynamic agents and has demonstrated efficacy and safety in a number of randomized controlled clinical trials as monotherapy and in combination with first-line antianginal therapies [18,19,20,21]. It is indicated as add-on therapy for the symptomatic treatment of stable angina in patients inadequately controlled or intolerant to first-line antianginal therapies. A once-daily TMZ formulation has recently become available that provides antianginal efficacy over 24 h [22, 23]. To date, there are no data on the efficacy and safety of this new formulation in combination with bisoprolol, the most widely prescribed beta-blocker for the treatment of stable angina [24]. The aim of the Modus Vivendi study was to address this evidence gap by evaluating the effect of TMZ 80 mg once daily (TMZ 80 OD) in patients with persistent symptoms despite treatment with maximally tolerated bisoprolol with or without other guideline-recommended antianginal therapies in routine clinical practice.
Methods
Modus Vivendi was a multicenter, prospective, observational, open-label, uncontrolled study conducted between October 2017 and July 2018 in Russian clinical practice. General practitioners and cardiologists with outpatient practices included adult patients > 18 years of age with a confirmed diagnosis of stable angina (defined as class II–III angina according to the Canadian Cardiovascular Society classification) to whose treatment they had decided to add TMZ 80 OD. At the start of the study, all patients were symptomatic despite optimal treatment according to guidelines for CCS management in force at the time of the study [25]. Optimization of antianginal therapy was at the discretion of the treating physician, but included agents for secondary prevention as well as at least one agent for symptom control. The treatment regimen had to include the maximally tolerated dose of bisoprolol. Enrollment was restricted to patients receiving bisoprolol as their beta-blocker component, for three reasons. First, bisoprolol is the most frequently prescribed beta-blocker worldwide, with prescription rates particularly high in Russia, where this study was conducted [24]. Second, both bisoprolol and TMZ 80 are both taken once daily. In real clinical practice, the ability to treat with two once-daily antianginal agents may result in improved adherence compared with agents that require twice-daily administration. The third reason was to provide data on the efficacy and safety of the once-daily formulation of TMZ 80 in combination with bisoprolol, which is currently lacking. Patients could also be receiving other antianginal drugs including calcium channel blockers, long-acting nitrates, and ivabradine. Guideline-recommended secondary prevention medications, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, and antithrombotic and antiplatelet agents, were also permitted and prescribed at the physician’s discretion.
Exclusion criteria were as follows: stable angina class IV or unstable angina within the past 6 months; a history of myocardial infarction or cerebrovascular accident within the past 3 months; uncontrolled hypertension (blood pressure greater than 180/100 mmHg) despite ongoing antihypertensive therapy; beta-blockers other than bisoprolol; inability to understand the nature of the program and follow the recommendations; pregnant or breast-feeding women; and any contraindications to the use of TMZ 80 OD.
The study was performed in accordance with good clinical practice and the ethical principles derived from the revised Declaration of Helsinki. Institutional Ethics Committee approval, provided by the Volvograd Regional Cardiology Center, was sought before performing the study, and all patients provided written informed consent. The study registration number is ISRCTN29992579.
Subjects were asked to make three visits to the study site: an inclusion visit (V1) at which patients were prescribed TMZ 80 mg once daily; a 1-month follow-up visit (V2); and a 3-month follow-up visit (V3). At each visit, the following information was collected: data on number of angina attacks and number of short-acting nitrate doses taken (based on patient diary); QoL assessments using the EuroQol 5-dimension 3-level (EQ-5D-3L) questionnaire [26, 27] and a visual analogue scale (VAS); and assessment of medication adherence. Information on spontaneously reported adverse drug reactions or events was collected at the V2 and V3 visits.
The EQ-5D-3L self-administered questionnaire comprises five dimensions: mobility, self-care, usual activities (for example, work, study, housework, participation in family affairs, leisure), pain/discomfort, and anxiety/depression. Each dimension has three response levels depending on the severity of the manifestation: no problems (score of 1), some problems (score of 2), and extreme problems (score of 3). QoL was also assessed using a 100 mm VAS on which 0 was the worst level of subjective perception of personal health and 100 was perfect health. Medication adherence was assessed using a non-validated questionnaire in Russian comprising six questions where the answer of “no” to all questions indicated good adherence, “yes” to one or two questions indicated moderate adherence, and “yes” to three or more questions indicated nonadherence.
As part of the study, two sub-analyses were performed to provide a clearer picture of whether the observed effects were due to the inclusion of TMZ 80 OD in the therapeutic regimen or as a result of background antianginal therapy. To achieve this, effectiveness was evaluated in the subgroup of patients whose background bisoprolol therapy remained constant throughout the study, and in the subgroup of patients with stable bisoprolol dose and whose background antianginal therapy was known.
Statistical Analysis
Data were analyzed using Statistical Analysis System (SAS) software (SAS Institute Inc., Cary, NC, USA). Parametric tests were used for variables with a normal distribution and nonparametric tests for variables with a non-normal distribution. Continuous (quantitative) data are presented using descriptive statistics including mean, standard deviation, and 95% confidence intervals. Qualitative or categorical variables are presented as absolute number and relative frequency of occurrence of each possible value.
When analyzing between-group differences for interval variables, the Student t-test values were calculated for independent samples using the corresponding formulas and in three different modifications that take into account the statistical distribution of a particular variable. The significance of the between-group changes in such variables during the treatment period was evaluated by the corresponding t-criteria for paired measurements.
In the case of binary variables, the significance of difference in the rate of detection of the factor in the two compared groups of patients was also evaluated by Student's t-test, but with the Fisher's arcsine transformation.
To assess the combined effect of different binary variables on the interval variable, analysis of variance (ANOVA) was used, and the significance of the effects was evaluated by Fisher's exact test.
The relationships between rank and binary variables were evaluated using contingency tables, and the significance of such relationships was assessed on the basis of three different modifications of the Chi-square test, Pearson test, and Fisher’s exact test.
Results
A total of 250 general practitioners and cardiologists recruited 1939 patients to take part in the observational study. Demographic and baseline clinical characteristics are shown in Table 1. The mean age was 65.6 ± 8.8 years. There were 829 (42.8%) men and 1110 (57.2%) women. The mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) values at V1 were 142.0 ± 14.9 mmHg and 84.5 ± 8.7 mmHg, respectively, and resting HR was 75.1 ± 9.5 bpm. Class II angina had been diagnosed in 73.8% of patients, and class III in 26.2%. The mean duration of CCS was 7.8 ± 6.4 years and the mean duration of angina was 7.1 ± 6.1 years.
Hypertension was present in 93.2% of patients, 85.5% had dyslipidemia, 28.5% had type 2 diabetes, 23.3% were current smokers, and 40.5% had a family history of CCS. A history of myocardial infarction was reported in 33.8%, percutaneous coronary intervention (PCI) in 20.4%, coronary artery bypass graft (CABG) in 18.2%, both PCI and CABG in 23.4%, and a history of stroke or transient ischemic attack in 12.7%.
At V1, 45.8% of patients were receiving a CCB, 24.6% long-acting nitrates (LAN), and 44.9% short-acting nitrates; the proportions of patients receiving antiplatelet agents and statins were 86.7% and 86.4%, respectively. Other medications prescribed during the study are illustrated in Table 1. In this observational study, a violation of the inclusion criteria (e.g. myocardial infarction within past 3 months, blood pressure greater than 180/100 mmHg despite antihypertensive therapy) was reported in 43 patients, and as a result, 1896 patients (97.8% of recruited patients) were included in the efficacy analyses. The starting doses of bisoprolol in the 1896 patients who completed the study are shown in Table 2.
Angina Attacks and Short-Acting Nitrate Use
At V1, the mean number of angina attacks per week was 6.2 ± 6.5 despite maximally tolerated bisoprolol therapy and use of other antianginal drugs. Following the addition of TMZ 80 OD to the treatment regimen this had decreased to 3.4 ± 4.2 attacks per week at V2 and 1.6 ± 2.6 at V3. Reductions were statistically significant between V1 and V2 and V1 and V3 (P < 0.05) (Table 3). The mean consumption of short-acting nitrates per week reflected the reduction in angina attacks and decreased from 4.9 ± 5.9 at V1 to 2.6 ± 3.8 at V2 and 1.1 ± 2.2 at V3 (Table 4). The reductions were statistically significant for V2 and V3 compared with baseline (P < 0.05).
Quality of Life
At V1 only 2.6% of patients reported no problems on all five dimensions of the EQ-5D-3L. At V2, the proportion reporting no problems using the EQ-5D-3L had increased to 15.7%, and at V3 it had increased to 44.0%. The improvement in all five dimensions of the EQ-5D-3L was statistically significant between V1 and V3 (P < 0.05). When analyzed with the VAS, mean QoL scores were 48.5 at V1, 61.7 at V2, and 75.7 at V3. The improvement in QoL score measured by the VAS was statistically significant between V1 and V2, and between V2 and V3 (P < 0.05).
Medication Adherence
The proportion of patients with “good adherence” to antianginal therapy increased over the course of the study, from 29.9% of patients at V1, to 41.7% at V2 and 53.8% at V3. The proportions of patients with “good or moderate adherence” at V1, V2, and V3 were 75.1%, 92.6%, and 96.3%, respectively.
Sub-analysis in Patients Who Maintained the Same Bisoprolol Dose Throughout the Study
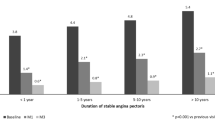
A sub-analysis was performed on the 1681 patients who received TMZ 80 OD and remained on the same maximally tolerated bisoprolol dose throughout the study. This included 292/312 (93.6%) who remained on 2.5 mg, 1005/1080 (93.1%) who remained on 5 mg, 323/339 (95.3%) who remained on 10 mg, and 61/165 (37.0%) who remained on “other” bisoprolol doses. At the baseline V1 visit, patients receiving the higher bisoprolol doses also had the greatest mean number of weekly angina attacks, which was as expected. Regardless of background bisoprolol dose, patients treated with TMZ 80 OD experienced statistically significant reductions in the number of weekly angina attacks after 1 month, which were maintained at the 3-month V3 visit (Fig. 1). Mean weekly consumption of short-acting nitrates reflected the pattern of weekly angina attacks, with the highest consumption in those with the greatest number of weekly episodes. Reductions in nitrate use were also statistically significant from 1 month and were maintained at V3 (Fig. 2).
Alleviation of angina symptoms was associated with significant improvements in QoL measured by both the EQ-5D-3L and VAS. These were observed for all domains and across all bisoprolol dose groups (Figs. 3 and 4).
Sub-analysis by Baseline Antianginal Therapy Among Patients Who Maintained the Same Bisoprolol Dose and Received TMZ 80 OD Throughout the Study
A total of 1610 patients remained on the same maximally tolerated bisoprolol dose and received TMZ OD 80 throughout the study. Of these 1610 patients, 680 were receiving bisoprolol monotherapy and TMZ 80 OD, while 930 were receiving bisoprolol plus other antianginal therapy (529 CCB, 187 LAN, and 214 both CCB and a LAN) and TMZ 80 OD. Statistically significant reductions from V1 in weekly anginal attacks and short-acting nitrate consumption were evident at both V2 and V3 in all groups (Figs. 5 and 6). Likewise, all groups showed improvement in QoL across all five domains of the EQ-5D-3L at both follow-up visits. Patients also reported a significant improvement in their health status on the 100-point VAS (Figs. 7 and 8). For all these endpoints, similar improvements were observed whether patients were treated with maximally tolerated bisoprolol and TMZ 80 OD alone, or maximally tolerated bisoprolol and TMZ 80 OD on top of background antianginal therapy.
Modifications to Background Antianginal Therapy
Background antianginal therapy was at the discretion of the investigator and could include CCB and/or LAN in addition to maximally tolerated bisoprolol and TMZ 80 OD. Among the 930 patients whose bisoprolol dose remained the same and who were taking TMZ 80 OD throughout the study, 529 were also receiving background therapy with a CCB, 187 with a LAN, and 214 with both a CCB and LAN (Table 5) The proportion of patients with no change to their background medication throughout the study was 98% in the CCB group and 85% in the LAN group. Among the patients receiving CCB and LAN background therapy, 85.5% of patients completed the study without a change in either medication, 94% completed without a change in CCB, and 85.5% completed without a change in LAN.
In very few cases there was a replacement of the LAN or CCB therapy. There were no patients with an increase in CCB dose, two patients had an increase in LAN dose, and in some cases the CCB or LAN therapy was stopped or the dose was reduced.
Safety and Tolerability
Safety data were collected for all patients who received at least one dose of TMZ 80 OD. During the course of the study there were no cases of hypotension or bradycardia. Two adverse events were recorded in two patients. The first was a circulatory arrest that occurred during coronary angiography in a patient who was not receiving TMZ 80 OD. The second was cardiac pain. Although the latter occurred in a patient receiving TMZ 80 OD, the event was not considered treatment-related.
Discussion
In patients with symptomatic, stable angina despite treatment with CCS guideline-recommended antianginal therapies including maximally tolerated bisoprolol, the addition of TMZ 80 OD to the therapeutic scheme reduced angina attacks and short-acting nitrate use. These findings were also observed in the subgroups of patients whose background bisoprolol therapy was unchanged throughout the study and in patients on stable bisoprolol dose receiving additional hemodynamic antianginal therapy in the form of a CCB and/or LAN. Reductions in clinical symptoms with the inclusion of TMZ 80 OD were also associated with improvements in QoL measured on two different scales, which assessed functional parameters such as mobility and ability to perform self-care, as well as overall health status.
There is consensus that it is reasonable to use combinations of classes of antianginal drugs for the treatment of anginal symptoms when symptoms persist despite the use of a single class [7]. However, regardless of whether beta-blockers are used alone or in combination, contemporary data from large registry studies such as CLARIFY and REACH have shown that a large proportion of patients with stable angina continue to experience ischemia and angina [13, 15, 24, 28]. Data from US outpatient cardiology practices for patients with a chronic CCS diagnosis have shown that around a quarter of patients continue to report monthly anginal symptoms, and 7.6% daily or weekly symptoms, despite over half (56.3%) being treated with at least two antianginal agents [14]. More recent data from the international CLARIFY registry reveal that of over 32,000 people with stable CCS, 7212 (22.1%) reported angina. Among these, 54.8% were still symptomatic for angina at 1 year despite management with at least two antianginal strategies including pharmacotherapy and revascularization [15]. Many patients also continue to experience anginal attacks despite revascularization [29,30,31,32]. In the ORBITA trial, 50.5% remained symptomatic after PCI despite a medical optimization phase during which the majority of patients received treatment with at least three antianginal agents, consisting predominantly of a beta-blocker, CCB, and LAN [32, 33].
A potential explanation for these findings is that the causes of symptomatic stable angina are multifactorial. More than one ischemic mechanism may coexist in the same patient, and these may change over a patient’s life span [34, 35]. When hemodynamic agents are not effective, the addition of a medication with an alternative mechanism of action may prove beneficial, instead of the addition of a further hemodynamic agent or increasing the dosages of those already prescribed. By shifting cardiac metabolism from free fatty acids towards glucose, TMZ is a promising approach for the treatment of patients with stable angina. By acting directly at the level of the ischemic cell, it bypasses the potential underlying vascular (macrovascular and/or microvascular) and hemodynamic mechanisms that can cause ischemia and angina.
In the ATPCI trial, TMZ 35 mg twice daily did not demonstrate superiority compared with placebo regarding the persistence or recurrence of angina leading to coronary angiography or intensification of antianginal therapy, although the trial included patients who had undergone successful, uncomplicated revascularization for stable angina or unstable angina/non-ST-elevation myocardial infarction (NSTEMI), where angina and ischemia were not an inclusion criterion [36]. Ischemia and angina were also not evaluated at baseline, nor was ischemia evaluation compulsory during follow-up, and thus the study design did not permit the objective evaluation of the anti-ischemic and antianginal efficacy of the drug in a population practically asymptomatic at inclusion. Of note, in the RIVER-PCI trial, ranolazine did not reduce rates of the composite primary efficacy endpoint of ischemia-driven revascularization or ischemia-driven hospitalization without revascularization, in spite of the fact that patients had incomplete revascularization [37] and 85% complained of angina at baseline [38].
In the current study, patients were symptomatic despite receiving maximally tolerated bisoprolol-based therapy, which in the majority of patients was represented by the 5 mg dose. Beta-blockers are often limited by side effects at higher doses, which can decrease patient adherence [39,40,41]. Data from the CLARIFY study have confirmed that in clinical practice, beta-blocker dosing is typically only 50% of the dose range required for demonstration of efficacy in randomized controlled trials [24]. As a cardioselective beta-blocker, bisoprolol is associated with superior efficacy and tolerability compared with many other members of this class [42], but side effects can remain dose-limiting in a number of patients.
Unlike conventional agents, the antianginal and anti-ischemic effects of TMZ are not associated with changes in hemodynamic determinants of myocardial oxygen consumption, such as heart rate, systolic blood pressure, or myocardial blood flow [43,44,45]. The efficacy of TMZ has been assessed in randomized, placebo-controlled studies, both as monotherapy and in combination with beta-blockers [19, 46, 47] and CCBs [48]. Comparator studies have shown that the efficacy of TMZ in stable angina is comparable to that of nifedipine and propranolol, with a lower incidence of side effects [43, 44], and twice as effective as LAN in patients symptomatic despite optimally tolerated beta-blocker therapy [46]. In clinical guidelines, beta-blockers or CCBs continue to be recommended as first-line agents [7], although no direct comparisons between first-choice and second-choice treatments have demonstrated the superiority of one group of drugs over the other [49]. Furthermore, no recommendations are made for the optimal combinations of drugs. Nevertheless, given the absence of effects on heart rate and blood pressure, TMZ represents an ideal agent for early combination therapy with conventional hemodynamic agents.
The current study adds to the growing body of literature supporting the use of TMZ in routine clinical practice [50,51,52], and in particular for the use of the once-daily formulation [23]. When TMZ 80 OD was added to the physician’s initial treatment, similar antianginal efficacy was observed at both 1 and 3 months, regardless of whether the background treatment was beta-blocker monotherapy or a dual or triple therapy combination, supporting findings from previous observational studies with both the twice-daily [52] and once-daily TMZ formulations [23].
To determine whether these observations were due to the inclusion of TMZ 80 OD or as a result of changes in the patients’ background antianginal therapy, a series of sub-analyses were performed. The first involved evaluation of antianginal efficacy among the subset of patients whose beta-blocker dose remained constant throughout the study. Statistically significant improvements in angina symptoms and QoL were observed regardless of beta-blocker dose. The second evaluated the efficacy of TMZ 80 OD among patients with stable beta-blocker dose receiving additional hemodynamic therapy (CCB and/or LAN). When TMZ was added to the physician’s initial treatment, similar improvements were observed regardless of whether the background treatment was beta-blocker monotherapy or a dual or triple therapy combination. During the study there were no statistically significant changes in the concomitant antianginal treatments or their doses. The benefits observed were therefore likely a result of the inclusion of TMZ 80 OD in the treatment regimen.
TMZ 80 OD was well tolerated and raised no unexpected safety concerns, including no reports of drug-induced neurotoxicity. The results of the recent ATPCI trial showed that the routine use of TMZ over several years in more than 6000 patients receiving optimal medical therapy after successful PCI was also not associated with any statistically significant safety concerns and demonstrated a safety profile comparable to that of placebo [36].
The beneficial effects of TMZ 80 OD were also obtained without adverse hemodynamic changes or an increased incidence of other side effects. In contrast, there are a number of limitations associated with the use of first-line agents in the context of the multifactorial nature of angina. Use of nonselective beta-blockers may worsen coronary vasospasm in some patients because blockade of the beta receptors, which mediate vasodilation, allows unopposed alpha receptor-mediated coronary vasoconstriction to occur [53]. Their use should therefore be avoided in patients with Prinzmetal angina. LAN have been suggested to cause endothelial dysfunction, via mechanisms such as the production of reactive oxygen species [54, 55], and worsen microvascular disease [56]. Both nitrates and CCB can also cause a phenomenon known as coronary steal, in which pharmacological vasodilation of coronary collateral arteries can shunt blood from ischemic to nonischemic zones, thereby potentially worsening ischemia [57].
Finally, in the current study, we observed an improvement in overall adherence to therapy and excellent tolerability. Treatment nonadherence, whether as a result of drug-related side effects or complexity of a dosing regimen, is known to contribute to suboptimal angina control [58,59,60]. It could be hypothesized that improvements in their clinical condition along with the once-daily regimen may have motivated patients to adhere to the medication, particularly given the well-established tolerability of TMZ 80 OD.
Study Limitations
This study was subject to the inherent limitations of observational studies, which include sample bias and incomplete response data, as well as the potential inaccuracy of self-reported behavior. Confirmation of the angina diagnosis with electrocardiography (ECG), stress testing with ECG or imaging, or coronary angiography was not a prerequisite of this study. Baseline coronary anatomy was not determined, and tests to determine exercise capacity before and after the introduction of TMZ 80 OD were not performed. The questionnaire used to assess medication adherence was non-validated, and therefore the results should be interpreted with caution. As an observational study without a control arm, blinding, or randomized treatment allocation, this study does not attempt to make any causal inferences about treatment effect. Understanding the characteristics of patients presenting with angina in routine clinical practice is, however, important as primary care is generally responsible for treatment continuity in most patients with CCS, and is where prescribing decisions are made. The findings from Modus Vivendi have important implications for the treatment of a wide range of patients with stable angina in clinical practice who still suffer frequent angina attacks despite guideline-recommended treatment.
Conclusion
The Modus Vivendi study results support the addition of TMZ 80 OD to first-line antianginal therapy to alleviate angina symptoms and improve QoL. Similar improvements in anginal symptoms and QoL were observed when TMZ 80 OD was added to maximally tolerated bisoprolol monotherapy, and when it was part of a treatment strategy with bisoprolol in double or triple combination with other hemodynamic agents such as CCBs and LANs. Our findings suggest that a maximally tolerated beta-blocker (bisoprolol) with add-on TMZ 80 OD is as effective as any of the other treatment combinations, and could represent an optimal first-line approach for the management of stable angina.
References
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858.
Blumenthal DM, Howard SE, Searl Como J, O’Keefe SM, Atlas SJ, Horn DM, Wagle NW, Wasfy JH, Yeh RW, Metlay JP. Prevalence of angina among primary care patients with coronary artery disease. JAMA Netw Open. 2021;4(6):e2112800.
Jespersen L, Abildstrøm SZ, Hvelplund A, Prescott E. Persistent angina: highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571–81.
Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129(14):1493–501.
Reynolds MW, Frame D, Scheye R, Rose ME, George S, Watson JB, Hlatky MA. A systematic review of the economic burden of chronic angina. Am J Manag Care. 2004;10(11 Suppl):S347–57.
Ye W, Su G, Miao W, Liu D, Yin L, Wang R, Xing Y, Lu Y, Lous S, Wu M, Yuan N, Xiong T. PCV6 model-based evaluation on annual economic burden of coronary atherosclerotic heart disease in China. Value Health Reg Issues. 2020;22(Suppl):S27.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Ferrari R, Pavasini R, Camici PG, Crea F, Danchin N, Pinto F, Manolis A, Marzilli M, Rosano GMC, Lopez-Sendon J, Fox K. Anti-anginal drugs-beliefs and evidence: systematic review covering 50 years of medical treatment. Eur Heart J. 2019;40(2):190–4.
Ferrari R, Ford I, Greenlaw N, et al. Geographical variations in the prevalence and management of cardiovascular risk factors in outpatients with CAD: data from the contemporary CLARIFY registry. Eur J Prev Cardiol. 2015;22(8):1056–65.
Sorbets E, Greenlaw N, Ferrari R, Ford I, Fox KM, Tardif JC, Tendera M, Steg PG, CLARIFY Investigators. Rationale, design, and baseline characteristics of the CLARIFY registry of outpatients with stable coronary artery disease. Clin Cardiol. 2017;40(10):797–806.
Steg PG, Ferrari R, Ford I, et al. Heart rate and use of beta-blockers in stable outpatients with coronary artery disease. PLoS ONE. 2012;7(5):e36284.
Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–9.
Steg PG, Greenlaw N, Tendera M, Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease (CLARIFY) Investigators. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the International Observational CLARIFY Registry. JAMA Intern Med. 2014;174:1651–9.
Kureshi F, et al. The prevalence and management of angina among patients with chronic coronary artery disease across US outpatient cardiology practices: insights from the angina prevalence and provider evaluation of angina relief (APPEAR) study. Clin Cardiol. 2017;40(1):6–10.
Mesnier J, Ducrocq G, Danchin N, Ferrari R, Ford I, Tardif JC, Tendera M, Fox KM, Steg PG, CLARIFY Investigators. International observational analysis of evolution and outcomes of chronic stable angina: the Multinational CLARIFY study. Circulation. 2021;144(7):512–23.
Balla C, Pavasini R, Ferrari R. Treatment of angina: where are we? Cardiology. 2018;140:52–67.
Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–8.
Chazov EI, et al. Trimetazidine in angina combination therapy – the TACT study: trimetazidine versus conventional treatment in patients with stable angina pectoris in a randomized, placebo-controlled, multicenter study. Am J Ther. 2005;12:35–42.
Szwed H, et al. Combination treatment in stable effort angina using trimetazidine and metoprolol: results of a randomized, double-blind, multicenter study (TRIMPOL II) TRIMetazidine in POLand. Eur Heart J. 2001;22:2267–74.
Danchin N, et al. Efficacy comparison of trimetazidine with therapeutic alternatives in stable angina pectoris: a network meta-analysis. Cardiology. 2011;120:59–72.
Peng S, Zhao M, Wan J, Fang Q, Fang D, Li K. The efficacy of trimetazidine on stable angina pectoris: a meta-analysis of randomized clinical trials. Int J Cardiol. 2014;177(3):780–5.
Pozdnyakov YM, et al. Clinical acceptability of trimetazidine modified-release 80 mg once daily versus trimetazidine modified-release 35 mg twice daily in stable angina pectoris. Cardiol Ther. 2018;7:61–70.
Glezer MG, et al. Anti-anginal effectiveness and tolerability of trimetazidine modified release 80mg once daily in stable angina patients in real-world practice. Adv Ther. 2018;35(9):1368–77.
Tendera M, Fox K, Ferrari R, Ford I, Greenlaw N, Abergel H, Macarie C, Tardif JC, Vardas P, Zamorano J, Gabriel Steg P, CLARIFY Registry Investigators. Inadequate heart rate control despite widespread use of beta-blockers in outpatients with stable CAD: findings from the international prospective CLARIFY registry. Int J Cardiol. 2014;176(1):119–24.
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Task Force Members. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003.
Dyer MTD, Goldsmith KA, Sharple LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13.
Euroqol. EQ-5D-3L. Available from: https://euroqol.org/eq-5d-instruments/eq-5d-3l-about.
Eisen A, Bhatt DL, Steg PG, Eagle KA, Goto S, Guo J, Smith SC, Ohman EM, Scirica BM, REACH Registry Investigators. Angina and future cardiovascular events in stable patients with coronary artery disease: insights from the reduction of atherothrombosis for continued health (REACH) registry. J Am Heart Assoc. 2016;5(10):e004080.
Stergiopoulos K, Boden WE, Hartigan P, Möbius-Winkler S, Hambrecht R, Hueb W, Hardison RM, Abbott JD, Brown DL. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med. 2014;174(2):232–40.
Ben-Yehuda O, Kazi DS, Bonafede M, Wade SW, Machacz SF, Stephens LA, Hlatky MA, Hernandez JB. Angina and associated healthcare costs following percutaneous coronary intervention: a real-world analysis from a multi-payer database. Catheter Cardiovasc Interv. 2016;88(7):1017–24.
Niccoli G, Montone RA, Lanza GA, Crea F. Angina after percutaneous coronary intervention: the need for precision medicine. Int J Cardiol. 2017;248:14–9.
Al-Lamee R, Howard JP, Shun-Shin MJ, Thompson D, Dehbi HM, Sen S, et al. Fractional flow reserve and instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease. Circulation. 2018;138(17):1780–92.
Foley M, Rajkumar CA, Shun-Shin M, Ganesananthan S, Seligman H, Howard J, et al. Achieving optimal medical therapy: insights from the ORBITA trial. J Am Heart Assoc. 2021;10(3):e017381.
Pepine CJ, Douglas PS. Rethinking stable ischemic heart disease: is this the beginning of a new era? J Am Coll Cardiol. 2012;60(11):957–9.
Kaski JC, Crea F, Gersh BJ, Camici PG. Reappraisal of ischemic heart disease. Circulation. 2018;138(14):1463–80.
Ferrari R, Ford I, Fox K, Challeton JP, Correges A, Tendera M, ATPCI investigators. Efficacy and safety of trimetazidine after percutaneous coronary intervention (ATPCI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396(10254):830–8.
Weisz G, Généreux P, Iñiguez A, Zurakowski A, Shechter M, Alexander KP, RIVER-PCI investigators. Ranolazine in patients with incomplete revascularisation after percutaneous coronary intervention (RIVER-PCI): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10014):136–45.
Alexander KP, Weisz G, Prather K, James S, Mark DB, Anstrom KJ, et al. Effects of ranolazine on angina and quality of life after percutaneous coronary intervention with incomplete revascularization: results from the Ranolazine for Incomplete Vessel Revascularization (RIVER-PCI) trial. Circulation. 2016;133(1):39–47.
Boyle RM, Bray CL, Naqvi N, Croxson RS, Cruickshank JM. A comparison of once and twice daily atenolol for angina pectoris. Int J Cardiol. 1983;3(1):25–35.
Gislason GH, Rasmussen JN, Abildstrøm SZ, Gadsbøll N, Buch P, Friberg J, Rasmussen S, Køber L, Stender S, Madsen M, Torp-Pedersen C. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27(10):1153–8.
Dimmitt SB, Stampfer HG, Warren JB. β-adrenoceptor blockers valuable but higher doses not necessary. Br J Clin Pharmacol. 2014;78(5):1076–9.
Sabidó M, Thilo H, Guido G. Long-term effectiveness of bisoprolol in patients with angina: a real-world evidence study. Pharmacol Res. 2019;139:106–12.
Dalla-Volta S, Maraglino G, Della-Valentina P, et al. Comparison of trimetazidine with nifedipine in effort angina: a double-blind, crossover study. Cardiovasc Drugs Ther. 1990;4(Suppl. 4):853–9.
Detry JM, Sellier P, Pennaforte S, et al. Trimetazidine: a new concept in the treatment of angina. Comparison with propranolol in patients with stable angina. Trimetazidine European Multicenter Study Group. Br J Clin Pharmacol. 1994;37:279–88.
Pornin M, Harpey C, Allal J, et al. Lack of effects of trimetazidine on systemic hemodynamics in patients with coronary artery disease: a placebo-controlled study. Clin Trials Metaanal. 1994;29:49–56.
Michaelidis AP, Spiropoulos K, Dimopoulos K, Athanasiades D, Toutouzas D. Antianginal efficacy of the combination of trimetazidine-propranolol compared with isosorbide dinitrate-propranolol in patients with stable angina. Clin Drug Invest. 1997;13(1):8–14.
Sellier P, Broustet JP. Assessment of anti-ischemic and antianginal effect at trough plasma concentration and safety of trimetazidine MR 35 mg in patients with stable angina pectoris: a multicenter, double-blind, placebo-controlled study. Am J Cardiovasc Drugs. 2003;3(5):361–9.
Manchanda SC, Krishnaswami S. Combination treatment with trimetazidine and diltiazem in stable angina pectoris. Heart. 1997;78(4):353–7.
Ferrari R, Camici PG, Crea F, Danchin N, Fox K, Maggioni AP, Manolis AJ, Marzilli M, Rosano GMC, Lopez-Sendon JL. Expert consensus document: a “diamond” approach to personalized treatment of angina. Nat Rev Cardiol. 2018;15(2):120–32.
Makolkin VI, Osadchiy KK. Trimetazidine modified release in the treatment of stable angina: TRIUMPH StudyTRImetazidine MR in patients with stable angina: unique metabolic PatH. Clin Drug Investig. 2004;24(12):731–8.
Nesukay EG. Treatment of stable angina in Ukraine: CLASSICA study. Ukrainian J Cardiol. 2014;2:43–7.
Glezer M, CHOICE-2 study investigators. Real-world evidence for the antianginal efficacy of trimetazidine from the Russian observational CHOICE-2 study. Adv Ther. 2017;34(4):915–24.
Kern MJ, Ganz P, Horowitz JD, Gaspar J, Barry WH, Lorell BH, Grossman W, Mudge GH Jr. Potentiation of coronary vasoconstriction by beta-adrenergic blockade in patients with coronary artery disease. Circulation. 1983;67:1178–85.
Knorr M, Hausding M, Kröller-Schuhmacher S, Steven S, Oelze M, Heeren T, et al. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and s-glutathionylation of endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2011;31(10):2223–31.
Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J Am Coll Cardiol. 2007;49(12):1289–95.
Tarkin JM, Kaski JC. Nicorandil and long-acting nitrates: vasodilator therapies for the management of chronic stable angina pectoris. Eur Cardiol. 2018;13(1):23–8.
Schulz W, Tatzel R, Jost S, Kaltenbach M, Kober G. Clinical Importance of the Coronary Steal Phenomenon: Are the Coronary Steal Phenomenon after Dipyridamole and the Antianginal Efficacy of Isosorbide Dinitrate or Nifedipine Related to Coronary Anatomy? In: Cohn JN, Rittinghausen R, editors. Mononitrates. Berlin, Heidelberg: International Boehringer Mannheim Symposia, Springer, Berlin, Heidelberg; 1985. https://doi.org/10.1007/978-3-642-70234-1_30.
Kronish IM, Ye S. Adherence to cardiovascular medications: lessons learned and future directions. Prog Cardiovasc Dis. 2013;55(6):590–600.
Kardas P, Investigators C. Comparison of once daily versus twice daily oral nitrates in stable angina pectoris. Am J Cardiol. 2004;94(2):213–6.
Ferdinand KC, Senatore FF, Clayton-Jeter H, Cryer DR, Lewin JC, Nasser SA, et al. Improving medication adherence in cardiometabolic disease: practical and regulatory implications. J Am Coll Cardiol. 2017;69(4):437–51.
Acknowledgements
The assistance of Yuriy Burtsev from Servier Russia in the preparation of this manuscript is gratefully acknowledged.
Funding
This study was funded by Servier, Russia. Editorial assistance for this paper was provided by Jenny Grice, Le Prioldy, France. This assistance and the journal’s Rapid Service Fee were funded by Servier, France.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors' contributions
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Yuri Lopatin has nothing to disclose relevant to the content of this article. Parvoleta Petrova is a Servier Global Medical and Patient Affairs employee.
Compliance with ethics guidelines
The study was performed in accordance with good clinical practice and the ethical principles derived from the revised Declaration of Helsinki. Institutional Ethics Committee approval, provided by the Volvograd Regional Cardiology Center, was sought before performing the study, and all patients provided written informed consent. The study registration number is ISRCTN29992579
Data availability
The datasets generated and/or analyzed for this study are not publicly available as they include medical records of patients from a secondary source.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lopatin, Y., Petrova, P. & the MODUS VIVENDI investigators. Effectiveness and Tolerability of Trimetazidine 80 Mg Once Daily in Patients with Stable Angina Uncontrolled with Bisoprolol-Based Therapy: The Modus Vivendi Observational Study. Cardiol Ther 11, 93–111 (2022). https://doi.org/10.1007/s40119-021-00249-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-021-00249-z