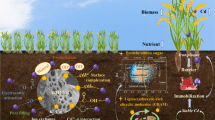
Abstract
In this study, enzyme-induced calcite precipitation (EICP)-based treatment for tropical soils contaminated with divalent heavy metals such as cadmium (Cd), nickel (Ni), and lead (Pb) were evaluated for their sorption and desorption capabilities. Heavy metals were taken in three different combinations: a single metal and a combination of two and three metals. They were mixed with locally available kaolinite and montmorillonite soils. EICP-treated soil retained the maximum quantity of Cd among all the heavy metals studied. The Cd retention exceeded Ni and Pb retention. The same was confirmed with desorption studies, relying on aggressive chelants such as ethylene diamine tetra acetic acid (EDTA) and citric acid. Before subjecting the kaolinitic soil to EICP treatment, it was found that the sorption capacity for Cd and Ni was 2.124 and 1.974 mg/g for Cd and Ni, respectively. The sorptive values increased to 3.457 and 4.418 mg/g for Cd and Ni, respectively, after EICP treatment. The retention is attributed to the formation of CdCO3 and NiCO3 in the soil matrix, which exhibits very low values of solubility product even in the presence of aggressive chelants. The study establishes that EICP treatment is a prospective method for remediation of soils laced with heavy metal ions.







Similar content being viewed by others
References
Roesch LFW, Fulthorpe RR, Riva A et al (2007) Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J 1:283–290. https://doi.org/10.1038/ismej.2007.53
Bardgett R (2005) The biology of soil: a community and ecosystem approach. Oxford University Press, Oxford, New York
Fierer N, Strickland MS, Liptzin D et al (2009) Global patterns in belowground communities. Ecol Lett 12:1238–1249. https://doi.org/10.1111/j.1461-0248.2009.01360.x
Wardle DA, Bardgett RD, Klironomos JN et al (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633. https://doi.org/10.1126/science.1094875
Moghal AAB, Sivapullaiah PV (2012) Retention characteristics of Cu2+, Pb2+, and Zn2+ from aqueous solutions by two types of low lime fly ashes. Toxicol Environ Chem 94:1941–1953. https://doi.org/10.1080/02772248.2012.732579
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226. https://doi.org/10.1016/j.biotechadv.2008.11.002
Vullo DL, Ceretti HM, Daniel MA et al (2008) Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E. Biores Technol 99:5574–5581. https://doi.org/10.1016/j.biortech.2007.10.060
Moghal AAB (2013) Geotechnical and physico-chemical characterization of low lime fly ashes. Adv Mater Sci Eng. https://doi.org/10.1155/2013/674306
Warren LA, Maurice PA, Parmar N, Ferris FG (2001) Microbially mediated calcium carbonate precipitation: implications for interpreting calcite precipitation and for solid-phase capture of inorganic contaminants. Geomicrobiol J 18:93–115. https://doi.org/10.1080/01490450151079833
Cai G-B, Zhao G-X, Wang X-K, Yu S-H (2010) Synthesis of polyacrylic acid stabilized amorphous calcium carbonate nanoparticles and their application for removal of toxic heavy metal ions in water. J Phys Chem C 114:12948–12954. https://doi.org/10.1021/jp103464p
Li M, Cheng X, Guo H, Yang Z (2016) Biomineralization of carbonate by terrabacter tumescens for heavy metal removal and biogrouting applications. J Environ Eng 142:C4015005. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000970
Al Qabany A, Soga K, Santamarina C (2012) Factors affecting efficiency of microbially induced calcite precipitation. J Geotech Geoenviron Eng 138:992–1001. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000666
Martinez BC, DeJong JT, Ginn TR et al (2013) Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J Geotech Geoenviron Eng 139:587–598. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000787
Rahman MM, Hora RN, Ahenkorah I et al (2020) State-of-the-art review of microbial-induced calcite precipitation and its sustainability in engineering applications. Sustainability 12:6281. https://doi.org/10.3390/su12156281
Martinez A, DeJong J, Akin I et al (2021) Bio-inspired geotechnical engineering: principles, current work, opportunities and challenges. Géotechnique. https://doi.org/10.1680/jgeot.20.P.170
D18 committee test methods for specific gravity of soil solids by water pycnometer ASTM International
Bureau of Indian Standards (1980) IS 2720–3–1: methods of test for soils, Part 3: determination of specific gravity, section 1: fine grained soils
D18 Committee Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International
Bureau of Indian Standards (1985) IS 2720–5: methods of test for soils, Part 5: determination of liquid and plastic limit
Bureau of Indian Standards (1972) IS 2720–6: Methods of test for soils, Part 6: Determination of shrinkage factors
D18 committee test method for pH of soils ASTM International
D18 committee test methods for moisture, ash, and organic matter of peat and other organic soils ASTM International
ASTM D1557 - 12e1 Standard test methods for laboratory compaction characteristics of soil using modified effort (56,000 FT-LBF - FT - Sup - 3 - Sup - (2,700 KN-M - M - Sup - 3 - Sup - ) ) | PDF | Soil | Materials. In: Scribd. https://www.scribd.com/document/367652171/ASTM-D1557-12e1-Standard-Test-Methods-for-Laboratory-Compaction-Characteristics-of-Soil-Using-Modified-Effort-56-000-Ft-lbf-ft-sup-3-sup-2-700-K. Accessed 13 Sep 2021
Bureau of Indian Standards (1992) IS 2720–9: methods of test for soils, Part 9: Determination of dry density- moisture content relation by constant mass of soil method
D18 Committee test method for unconfined compressive strength of cohesive soil ASTM International
Bureau of Indian Standards (1991) IS 2720–10: methods of test for soils, Part 10: determination of unconfined compressive strength
Knorr B (2014) Enzyme-induced carbonate precipitation for the mitigation of fugitive dust. Dissertation, Arizona State University
Yasuhara H, Neupane D, Hayashi K, Okamura M (2012) Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found 52:539–549. https://doi.org/10.1016/j.sandf.2012.05.011
Krajewska B (2018) Urease-aided calcium carbonate mineralization for engineering applications: a review. J Adv Res 13:59–67. https://doi.org/10.1016/j.jare.2017.10.009
Blakeley RL, Zerner B (1984) Jack bean urease: the first nickel enzyme. J Mol Catal 23:263–292. https://doi.org/10.1016/0304-5102(84)80014-0
Achal V, Pan X (2014) Influence of calcium sources on microbially induced calcium carbonate precipitation by Bacillus sp. CR2. Appl Biochem Biotechnol 173:307–317. https://doi.org/10.1007/s12010-014-0842-1
Almajed A, Tirkolaei HK, Kavazanjian E, Hamdan N (2019) Enzyme induced biocementated sand with high strength at low carbonate content. Sci Rep 9:1135. https://doi.org/10.1038/s41598-018-38361-1
Mohammed SAS, Moghal AAB (2014) Soils amended with admixtures as stabilizing agent to retain heavy metals 2216–2225 https://doi.org/10.1061/9780784413272.216
Mohammed SAS, Moghal AAB, Sanaulla PF, et al (2017) Cadmium fixation studies on contaminated soils using nano calcium silicate—treatment strategy 434–442 https://doi.org/10.1061/9780784480434.047
Moghal AAB, Lateef MA, Mohammed SAS et al (2020) Efficacy of enzymatically induced calcium carbonate precipitation in the retention of heavy metal ions. Sustainability 12:7019. https://doi.org/10.3390/su12177019
Gu Y-Y, Yeung AT (2011) Desorption of cadmium from a natural Shanghai clay using citric acid industrial wastewater. J Hazard Mater 191:144–149. https://doi.org/10.1016/j.jhazmat.2011.04.054
Khodadoust AP, Reddy KR, Maturi K (2004) Removal of nickel and phenanthrene from kaolin soil using different extractants. Environ Eng Sci 21:691–704. https://doi.org/10.1089/ees.2004.21.691
ASTM D3987 - 12 Standard practice for shake extraction of solid waste with water ASTM International, West Conshohocken, PA, USA
Moghal AAB, Reddy KR, Mohammed SAS et al (2017) Sorptive Response of chromium (Cr+6) and mercury (Hg+2) from aqueous solutions using chemically modified soils. JTE 45:105–119. https://doi.org/10.1520/JTE20160066
ASTM D4646 - 16 (2016) Test method for 24-h batch-type measurement of contaminant sorption by soils and sediments ASTM International: West Conshohocken, PA, USA
Moghal AAB, Ashfaq M, Al-Shamrani MA, Al-Mahbashi A (2020) Effect of heavy metal contamination on the compressibility and strength characteristics of chemically modified semiarid soils. J Hazard Toxic Radioact Waste 24:04020029. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000527
Dixit R, Wasiullah MD et al (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7:2189–2212. https://doi.org/10.3390/su7022189
Kapoor A, Viraraghavan T (1995) Fungal biosorption — an alternative treatment option for heavy metal bearing wastewaters: a review. Biores Technol 53:195–206. https://doi.org/10.1016/0960-8524(95)00072-M
Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59:203–216. https://doi.org/10.1016/S0304-386X(00)00160-2
Karageorgiou K, Paschalis M, Anastassakis GN (2007) Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J Hazard Mater 139:447–452. https://doi.org/10.1016/j.jhazmat.2006.02.038
Godelitsas A, Astilleros JM, Hallam K et al (2003) Interaction of calcium carbonates with lead in aqueous solutions. Environ Sci Technol 37:3351–3360. https://doi.org/10.1021/es020238i
Habte L, Shiferaw N, Khan MD et al (2020) Sorption of Cd2+ and Pb2+ on aragonite synthesized from eggshell. Sustainability 12:1174. https://doi.org/10.3390/su12031174
Fraser A, Lambkin DC, Lee MR et al (2011) Incorporation of lead into calcium carbonate granules secreted by earthworms living in lead contaminated soils. Geochim Cosmochim Acta 75:2544–2556. https://doi.org/10.1016/j.gca.2011.02.015
Makuchowska-Fryc J (2019) Use of the eggshells in removing heavy metals from waste water - the process kinetics and efficiency. Ecol Chem Eng S 26:165–174. https://doi.org/10.1515/eces-2019-0012
Sdiri A, Higashi T (2013) Simultaneous removal of heavy metals from aqueous solution by natural limestones. Appl Water Sci 3:29–39. https://doi.org/10.1007/s13201-012-0054-1
Moghal AAB, Mohammed SAS, Al-Shamrani MA, Zahid WM (2014) Performance of soils and soil lime mixtures as liners to retain heavy metal ions in aqueous solutions 160–169 https://doi.org/10.1061/9780784413432.017
Zhu X, Kumari D, Huang M, Achal V (2016) Biosynthesis of CdS nanoparticles through microbial induced calcite precipitation. Mater Des 98:209–214. https://doi.org/10.1016/j.matdes.2016.03.008
Nancharaiah YV, Lens PNL (2015) Selenium biomineralization for biotechnological applications. Trends Biotechnol 33:323–330. https://doi.org/10.1016/j.tibtech.2015.03.004
Zachara JM, Cowan CE, Resch CT (1991) Sorption of divalent metals on calcite. Geochim Cosmochim Acta 55:1549–1562. https://doi.org/10.1016/0016-7037(91)90127-Q
Tang Y, Elzinga EJ, Jae Lee Y, Reeder RJ (2007) Coprecipitation of chromate with calcite: batch experiments and X-ray absorption spectroscopy. Geochim Cosmochim Acta 71:1480–1493. https://doi.org/10.1016/j.gca.2006.12.010
Elzinga EJ, Rouff AA, Reeder RJ (2006) The long-term fate of Cu2+, Zn2+, and Pb2+ adsorption complexes at the calcite surface: an X-ray absorption spectroscopy study. Geochim Cosmochim Acta 70:2715–2725. https://doi.org/10.1016/j.gca.2006.02.026
Merdy P, Gharbi LT, Lucas Y (2009) Pb, Cu and Cr interactions with soil: sorption experiments and modelling. Colloids Surf, A 347:192–199. https://doi.org/10.1016/j.colsurfa.2009.04.004
Seco A, Marzal P, Gabaldón C, Ferrer J (1997) Adsorption of heavy metals from aqueous solutions onto activated carbon in single Cu and Ni systems and in binary Cu–Ni, Cu–Cd and Cu–Zn systems. J Chem Technol Biotechnol 68:23–30. https://doi.org/10.1002/(SICI)1097-4660(199701)68:1%3c23::AID-JCTB595%3e3.0.CO;2-N
Soares MAR, Quina MJ, Quinta-Ferreira RM (2015) Immobilisation of lead and zinc in contaminated soil using compost derived from industrial eggshell. J Environ Manag 164:137–145. https://doi.org/10.1016/j.jenvman.2015.08.042
Fleming M, Tai Y, Zhuang P, McBride MB (2013) Extractability and bioavailability of Pb and As in historically contaminated orchard soil: effects of compost amendments. Environ Pollut 177:90–97. https://doi.org/10.1016/j.envpol.2013.02.013
Kang C-H, Kwon Y-J, So J-S (2016) Bioremediation of heavy metals by using bacterial mixtures. Ecol Eng 89:64–69. https://doi.org/10.1016/j.ecoleng.2016.01.023
Achal V, Pan X, Zhang D, Fu Q (2012) Bioremediation of Pb-contaminated soil based on microbially induced calcite precipitation. J Microbiol Biotechnol 22(2):244-247. https://doi.org/10.4014/jmb.1108.08033
GarcÍa-Sánchez A, Álvarez-Ayuso E (2002) Sorption of Zn, Cd and Cr on calcite. Application to purification of industrial wastewaters. Minerals Eng 15:539–547. https://doi.org/10.1016/S0892-6875(02)00072-9
Al-Degs YS, El-Barghouthi MI, Issa AA et al (2006) Sorption of Zn(II), Pb(II), and Co(II) using natural sorbents: equilibrium and kinetic studies. Water Res 40:2645–2658. https://doi.org/10.1016/j.watres.2006.05.018
Makino T, Sugahara K, Sakurai Y et al (2006) Remediation of cadmium contamination in paddy soils by washing with chemicals: Selection of washing chemicals. Environ Pollut 144:2–10. https://doi.org/10.1016/j.envpol.2006.01.017
Kumari D, Qian XY, Pan X, Achal V, Li Q, Gadd GM (2016) Microbially-induced carbonate precipitation for immobilization of toxic metals. Adv Appl Microbiol 94:79–108. https://doi.org/10.1016/bs.aambs.2015.12.002
O’Connor GA, O’Connor C, Cline GR (1984) Sorption of cadmium by calcareous soils: influence of solution composition. Soil Sci Soc Am J 48:1244–1247. https://doi.org/10.2136/sssaj1984.03615995004800060008x
Campillo-Cora C, Conde-Cid M, Arias-Estévez M et al (2020) Specific adsorption of heavy metals in soils: individual and competitive experiments. Agronomy 10:1113. https://doi.org/10.3390/agronomy10081113
Li W, Zhang Y, Achal V (2022) Mechanisms of cadmium retention on enzyme-induced carbonate precipitation (EICP) of Ca/Mg: Nucleation, chemisorption, and co-precipitation. J Environ Chem Eng 10:107507. https://doi.org/10.1016/j.jece.2022.107507
Goldberg S, Criscenti LJ, Turner DR, et al. (2007) Adsorption-desorption processes in subsurface reactive transport modeling https://doi.org/10.2136/vzj2006.0085
Blais JF, Djedidi Z, Cheikh RB et al (2008) Metals precipitation from effluents: review. Pract Periodical Hazard Toxic Radioact Waste Manag 12:135–149. https://doi.org/10.1061/(ASCE)1090-025X(2008)12:3(135)
Moghal AAB, Reddy KR, Mohammed SAS et al (2016) Lime-amended semi-arid soils in retaining copper, lead, and zinc from aqueous solutions. Water Air Soil Pollut 227:372. https://doi.org/10.1007/s11270-016-3054-1
Mohammed SAS, Moghal AAB (2016) Efficacy of nano calcium silicate (NCS) treatment on tropical soils in encapsulating heavy metal ions: leaching studies validation. Innov Infrastruct Solut 1:21. https://doi.org/10.1007/s41062-016-0024-9
Madhu A, Chakraborty JN (2017) Developments in application of enzymes for textile processing. J Clean Prod 145:114–133. https://doi.org/10.1016/j.jclepro.2017.01.013
Dejong JT, Soga K, Kavazanjian E et al (2013) Biogeochemical processes and geotechnical applications: progress, opportunities and challenges. Géotechnique 63:287–301. https://doi.org/10.1680/geot.SIP13.P.017
Vega FA, Covelo EF, Andrade ML (2008) A versatile parameter for comparing the capacities of soils for sorption and retention of heavy metals dumped individually or together: results for cadmium, copper and lead in twenty soil horizons. J Colloid Interface Sci 327:275–286. https://doi.org/10.1016/j.jcis.2008.08.027
Acosta JA, Jansen B, Kalbitz K et al (2011) Salinity increases mobility of heavy metals in soils. Chemosphere 85:1318–1324. https://doi.org/10.1016/j.chemosphere.2011.07.046
González Costa JJ, Reigosa MJ, Matías JM, Covelo EF (2017) Soil Cd, Cr, Cu, Ni, Pb and Zn sorption and retention models using SVM: variable selection and competitive model. Sci Total Environ 593–594:508–522. https://doi.org/10.1016/j.scitotenv.2017.03.195
Wang YM, Chen TC, Yeh KJ, Shue MF (2001) Stabilization of an elevated heavy metal contaminated site. J Hazard Mater 88:63–74. https://doi.org/10.1016/S0304-3894(01)00289-8
Mohammed SAS, Sanaulla PF, Moghal AAB (2016) Sustainable use of locally available red earth and black cotton soils in retaining Cd2+ and Ni2+ from aqueous solutions. Int J Civ Eng 14:491–505. https://doi.org/10.1007/s40999-016-0052-z
Zhou DM, Wang SQ, Chen HM (2001) Interaction of Cd and citric acid, EDTA in red soil. J Environ Sci 13(2):153–156
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments – a review. Waste Manag 28:215–225. https://doi.org/10.1016/j.wasman.2006.12.012
Manjeet B, Diwan S, Garg VK, Pawan R (2009) Use of agricultural waste for the removal of nickel ions from aqueous solutions: equilibrium and kinetics studies. Int J Environ Sci Eng 1(2):108–114. https://doi.org/10.5281/zenodo.1075328
Attar K, Demey H, Bouazza D, Sastre AM (2019) Sorption and desorption studies of Pb(II) and Ni(II) from aqueous solutions by a new composite based on alginate and magadiite materials. Polymers 11:340. https://doi.org/10.3390/polym11020340
Karna RR, Luxton T, Bronstein KE et al (2017) State of the science review: Potential for beneficial use of waste by-products for in situ remediation of metal-contaminated soil and sediment. Crit Rev Environ Sci Technol 47:65–129. https://doi.org/10.1080/10643389.2016.1275417
Li C, Qian Z, Zhou C et al (2014) Mussel-inspired synthesis of polydopamine-functionalized calcium carbonate as reusable adsorbents for heavy metal ions. RSC Adv 4:47848–47852. https://doi.org/10.1039/C4RA08193E
Peters RW (1999) Chelant extraction of heavy metals from contaminated soils. J Hazard Mater 66:151–210. https://doi.org/10.1016/S0304-3894(99)00010-2
Cline SR, Reed BE (1995) Lead removal from soils via bench-scale soil washing techniques. J Environ Eng 121:700–705. https://doi.org/10.1061/(ASCE)0733-9372(1995)121:10(700)
Kavazanjian E, Almajed A, Hamdan N (2017) Bio-inspired soil improvement using EICP soil columns and soil nails https://doi.org/10.1061/9780784480793.002
Ran D, Kawasaki S (2016) Effective use of plant-derived urease in the field of geoenvironmental/ geotechnical engineering. J Civil Environ Eng 6:1–13. https://doi.org/10.4172/2165-784X.1000207
Dhami NK, Reddy MS, Mukherjee A (2013) Biomineralization of calcium carbonates and their engineered applications: a review. Front Microbiol. https://doi.org/10.3389/fmicb.2013.00314
Madrid L, Diaz-Barrientos E (1992) Influence of carbonate on the reaction of heavy metals in soils. J Soil Sci 43:709–721. https://doi.org/10.1111/j.1365-2389.1992.tb00170.x
Torres-Aravena ÁE, Duarte-Nass C, Azócar L et al (2018) Can microbially induced calcite precipitation (MICP) through a ureolytic pathway be successfully applied for removing heavy metals from wastewaters? Crystals 8:438. https://doi.org/10.3390/cryst8110438
Nathan VK, Rani ME, Gunaseeli R, Kannan ND (2018) Enhanced biobleaching efficacy and heavy metal remediation through enzyme mediated lab-scale paper pulp deinking process. J Clean Prod 203:926–932. https://doi.org/10.1016/j.jclepro.2018.08.335
Han L, Li J, Xue Q et al (2020) Bacterial-induced mineralization (BIM) for soil solidification and heavy metal stabilization: a critical review. Sci Total Environ 746:140967. https://doi.org/10.1016/j.scitotenv.2020.140967
Li M, Cheng X, Guo H (2013) Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int Biodeterior Biodegrad 76:81–85. https://doi.org/10.1016/j.ibiod.2012.06.016
Govarthanan M, Lee K-J, Cho M et al (2013) Significance of autochthonous Bacillus sp. KK1 on biomineralization of lead in mine tailings. Chemosphere 90:2267–2272. https://doi.org/10.1016/j.chemosphere.2012.10.038
Noel JD, Biswas P, Giammar DE (2007) Evaluation of a sequential extraction process used for determining mercury binding mechanisms to coal combustion byproducts. J Air Waste Manag Assoc 57:856–867. https://doi.org/10.3155/1047-3289.57.7.856
Kotresha K, Mohammed SAS, Sanaulla PF et al (2020) Evaluation of sequential extraction procedure (SEP) to validate binding mechanisms in soils and soil-nano-calcium silicate (SNCS) mixtures. Indian Geotech J. https://doi.org/10.1007/s40098-020-00464-w
Acknowledgements
This project was financially supported by the National Institute of Technology, Warangal, India, under “Research Seed Grant No: NITW/AC-7/RSM-Bdgt/2018-2019/P1015” and the Ministry of Education (formerly known as Ministry of Human Resource and Development), Government of India. The authors thank the reviewers for their constructive comments which helped the cause of the manuscript.
Funding
This project was financially supported by the National Institute of Technology, Warangal, India, under “Research Seed Grant No: NITW/AC-7/RSM-Bdgt/2018-2019/P1015” and the Ministry of Education (formerly known as Ministry of Human Resource and Development), Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moghal, A.A.B., Rasheed, R.M. & Mohammed, S.A.S. Sorptive and Desorptive Response of Divalent Heavy Metal Ions from EICP-Treated Plastic Fines. Indian Geotech J 53, 315–333 (2023). https://doi.org/10.1007/s40098-022-00638-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40098-022-00638-8