Abstract
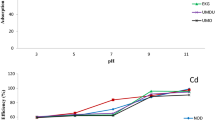
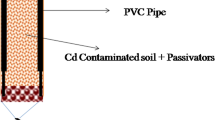
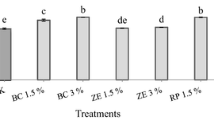
The relative performance of two soils as a sustainable material to attenuate the transport of heavy metal ions, cadmium (Cd2+) and nickel (Ni2+), from aqueous solutions has been evaluated. Red earth soil (RS) and black cotton soil (BCS) originating from India, were selected, and batch equilibrium tests including sorption kinetics and leaching studies were conducted. Langmuir isotherm was found to be more suitable than Freundlich isotherm for both the soils. Kinetic data were fitted on four models namely pseudo first order, pseudo second order, Elovich and intraparticle diffusion. Correlation coefficients obtained by all models fitted well in the following ranking: Elovich > Intraparticle diffusion > pseudo second order > pseudo first order. Based on extensive experimental data, it is concluded that the ranking on sorption was of the order Cd > Ni for both the soils, and BCS exhibited relatively higher retention levels compared to RS. It is further concluded that BCS can be used as a substitute to filter material, RS a substitute to main liner material in attenuating Cd2+ and Ni2+ from an industrial landfill leachate.











Similar content being viewed by others
References
Sivapullaiah PV, Moghal AAB (2010) Leachability of trace elements from two stabilized low lime indian fly ashes. Environ Earth Sci 61:1735–1744
Sungur A, Soylak M, Yilmaz E, Yilmaz S, Ozcan H (2015) Characterization of heavy metal fractions in agricultural soils by sequential extraction procedure: the relationship between soil properties and heavy metal fractions. J Soil Sed Cont 24(1):1–15
ATSDR (Agency for toxic substances and disease registry)(2012), Toxicological profile for Cadmium–potential for human exposure: accessed November 11th, 2014; http://www.atsdr.cdc.gov/toxprofiles/tp5-c6.pdf/
ATSDR (Agency for toxic substances and disease registry)(2005). Toxicological profile for Nickel—potential for human exposure: accessed November 11th, 2014; http://www.atsdr.cdc.gov/toxprofiles/tp15-c6.pdf/
Heike BB (2004) Adsorption of Heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1–18
Gadepalle VP, Ouki SK, Herwijnen RV, Hutchings T (2007) Immobilization of heavy metals in soil using natural and waste materials for vegetation establishment on contaminated sites. J Soil Sed Cont 16(2):233–251
Sivapullaiah PV, Moghal AAB (2011) Gypsum treated fly ash as a liner for waste disposal facilities. J Waste Manag 31:359–369
Ho YS, Mc Kay G (1999) Pseudo-second order model for sorption processes. J Pro Biochem 34:451–465
Moghal AAB, Mohammed SAS, Basha BM, Shamrani MAA (2014) Surface complexation modeling for stabilization of an industrial sludge by alternate materials. Geotech Spec Publ 234:2235–2244
Veli S, Alyuz B (2007) Adsorption of Zinc and zinc from aqueous solutions by using natural clay. J Hazard Mat 149:226–233
McKinley JD, Thomas HR, Williams KP, Reid JM (2001) Chemical analysis of contaminated soil strengthened by the addition of lime. J Eng Geo 60(1–4):181–192
Wilkinson A, Haque A, Kodikara J (2010) Stabilisation of clayey soils with industrial by-products: part A. Proc Inst Civ Eng Ground Improv 163(3):149–163
Falamaki A (2013) Artificial neural network application for predicting soil distribution coefficient of nickel. J Environ Radioact 115:6–12
Falamaki A, Tavallali H, Eskandari M, Farahmand SR (2016) Immobilizing some heavy metals by mixing contaminated soils with phosphate admixtures. Int J Civ Eng. doi:10.1007/s40999-016-0006-5
Moghal AAB, Mohammed SAS, Shamrani MAA, Zahid WM (2014) Performance of soils and soil lime mixtures as liners to retain heavy metal ions in aqueous solutions. Geotech Spec Publ 241:160–169
Niu ZX, Sun LN, Sun TH (2011) The bioadsorption of cadmium and lead by bacteria in root exudates culture. J Soil Sed Cont 20(8):877–891
Liu HB, Guo PT, Wei W, Wang ZY (2011) Assessment of soil arsenic, chromium, mercury, and lead at an agricultural landscape scale. J Soil Sed Cont 20(8):995–1007
ASTM (American Standard Testing Methods) (2008). Standard Test Method for 24-h Batch-Type Measurement of Contaminant Sorption by Soils and Sediments D4646. West Conshohocken, PA
ASTM (American Standard Testing Methods) (2012). Standard Practice for Shake Extraction of Solid Waste with Water D3987. West Conshohocken, PA
Harter RD (1983) Effect of soil pH on adsorption of lead, copper, zinc, and nickel. J Soil Sci Soc Am 47(1):47–51
Gherbi N, Meniai AH, Bencheikh M, Mansri A, Morcellet M, Bellir K, Bacquet M, Martel B (2004) Study of retention phenomenon of copper II by calcinated wheat by products. J Desalination 166:363–369
Moghal AAB, Reddy KR, Mohammed SAS, Shamrani MAA, Zahid WM (2017) Retention studies on arsenic from aqueous solutions by lime treated semi arid soils. Int J Geomate 12(29):2836–2843
Pérez AM, Paradelo M, Munoz JC, Estevez MA, Sanjurjo MJF, Rodriguez EA, Delgado AN (2013) Heavy metal retention in copper mine soil treated with mussel shells: batch and column experiments. J Hazard Mat 248–249:122–130
Moirou A, Xenidis A, Paspaliaris I (2001) Stabilization Pb, Zn, and Cd-contaminated soil by means of natural zeolite. J Soil Sed Cont 10(3):251–267
Coles CA, Yong RN (2006) Use of equilibrium and initial metal concentrations in determining Freundlich isotherms for soils and sediments. J Eng Geo 85:19–25
Nemr A (2009) Potential of pomegranate husk carbon for Cr(VI) removal from wastewater: kinetic and Isotherm studies. J Hazard Mat 161:132–141
Moghal AAB, Shamrani MAA, Zahid WM (2015) heavy metal desorption studies on the artificially contaminated Al-Qatif soil. Int J Geomate 8(2):1323–1327
Sawell SE, Bridle TR, Constable TW (1988) Heavy metal leachability from solid waste incinerator ashes. J Waste Manag Res 6(3):227–238
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite, a review. Adv Coll Interface Sci 140(2):114–131
Tossavainen M, Forssberg E (1999) The potential leachability from natural road construction materials. J Sci Total Environ 239(1–3):31–47
Moghal AAB, Sivapullaiah PV (2012) Retention characteristics of Cu2+, Pb2+ and Zn2+ from aqueous solutions by two types of low lime fly ashes. J Toxic Environ Chem 94–10:1941–1953
Covelo EF, Vega FA, Andrade ML (2007) Heavy metal sorption and desorption capacity of soils containing endogenous contaminants. J Hazard Mat 143(1–2):419–430
Bishop PL (1998) Leaching of inorganic hazardous constituents from stabilized/solidified hazardous wastes. J Hazard Waste Hazard Mat 5(2):129–143
Mohammed SAS, Moghal AAB (2014) Soils amended with admixtures as stabilizing agent to retain heavy metals. Geotech Spec Publ 234:2216–2226
Meima J, Comans R (1998) Application of surface complexation/precipitation modeling to contaminant leaching from weathered municipal solid waste incinerator bottom ash. J Environ Sci Tech 32:688–695
Usman ARA (2008) The relative adsorption selectivities of Pb, Cu, Zn, Cd and Ni by soils developed on shale in New Valley, Egypt. J Geoderma 144(1–2):334–343
Cappuyns V, Swennen R (2008) The application of pH state leaching tests to assess the pH-dependent release of trace metals from soils, sediments and waste material. J Hazard Mat 158(1):185–195
Acknowledgments
This paper is part of a research project supported by Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Govt. of India, Project No. SR/S3/MERC/0111/2012. The authors would also like to thank Mr. Kotresha K, Junior Research Fellow (JRF) for his assistance in conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammed, S.A.S., Sanaulla, P.F. & Moghal, A.A.B. Sustainable Use of Locally Available Red Earth and Black Cotton Soils in Retaining Cd2+ and Ni2+ from Aqueous Solutions. Int J Civ Eng 14, 491–505 (2016). https://doi.org/10.1007/s40999-016-0052-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40999-016-0052-z