Abstract
In this work to investigate the effects of using different gases in cold atmospheric plasma (CAP) on surface treatment of silver thin films (100, 120 nm film thickness) He and Ar were employed. Ag was sputtered on glass substrate and X-ray diffraction (XRD) as well as atomic force microscopy (AFM) for structural and morphological studies before and after plasma radiation was carried out. Surface treatment of Ag thin films by CAP increased surface roughness especially with Ar plasma, this rise of surface roughness of Ag thin film leads to make their surface hydrophilic which can be very important for antibacterial activity of Ag films in removal of Escherichia coli (E. coli) which was used as target bacterium. Argon plasma radiation was more effective than helium on both surface morphology and anti-bacterial property. XRD results showed that crystalline orientation of [111] peak of silver remains as preferred orientation, but the grain size did not change that much after plasma treatment. The crystalline size was calculated by Debye–Scherrer formula using peak [111] of Ag samples (as the most intense peak of silver).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For many decades silver and gold are known as the best electrical conductors and Ag specially has various applications in different sciences and technologies [1]. Silver thin films have been employed for surface plasmon resonance (SPR) which has many usages especially in biology, medical sciences and environment. Some nano particles such as Ag, Cu and ZnO have attracted much interest due to their unique optical and antibacterial properties [2, 3]. Nowadays, humans are looking for low cost and high efficiency methods for treating wastewater and sweetening water especially due to lack of safe and pure drinking water, Ag with anti-bacterial activity can be very helpful [3, 4]. Microorganisms are part of the organic materials which are commonly present in the wastewater, which can affect human life. Often the removal of pathogenic microorganisms is the final step in the wastewater treatment. Antibacterial activity of silver in nanoscale is higher and is more reactive due to large surface area to volume ratio [5, 6]. In recent decades due to growth of population and rise of water consumption shortage of pure water (especially with bacterial and microbial problems) have caused a lots of difficulties for human. For this reason the main purpose of this study was concentrated on investigation of anti-microbial property of Ag thin films in water after using plasma treatment on their surfaces [4,5,6]. He and Ar were utilized for plasma jet and the obtained results were compared. Silver thin films can be produced by different methods such as chemical vapor deposition (CVD), sol–gel, physical vapor deposition (PVD), thermal and electron gun beam evaporation, laser deposition, RF, DC sputtering, etc. [7,8,9,10]. In this experimental work magnetron sputtering was employed, since it has many advantages such as low temperature (no agglomeration), high purity, suitable and controllable coating rate. Using this method of deposition a better adhesion of thin film to substrate due to higher energy of condensing particles can be obtained, also annealing and post-annealing can lead to better adhesion and with sputtering large substrates may be used which can be good for industrial purposes [9,10,11]. In our previous works silver and copper and ZnO (as antibacterial materials) were employed in fabrication and modification of different polymeric membranes for separating salt, heavy and valuable metals from waters and also their antibacterial activity were studied (but low amount of Ag in membranes was not sufficient and not very effective for removal of bacterium). Since they were very thin (micrometer thickness) and may not resist high pressure from industrial wastewater for removal of microbial impurities so they were good only for dead end systems (static waters) [12, 13]. In the past few years non-thermal plasma has been used in various technologies, sciences and recently in medical instruments due to its anti-microbial property and it has been employed in wound healing, special medical bandage, and removing infections [14,15,16,17,18].
Cold atmospheric plasma (CAP) has been used to improve different surfaces for various materials (di-electric, membranes, different polymers, and metals, etc.) and has different applications such as changing hydrophilic or hydrophobic property and varying surface roughness and sometimes warm or hot plasma are used for hardening and smoothing surfaces from friction and erosion point of view [18,19,20,21]. Obviously these effects depend on type of used gases like (O2, N2, Ar, He, H2, …, etc.), some times more than one gas is used and the second or third gas is employed with partial pressure or as assisted gas depending on the purpose of using plasma [22, 23]. Since both silver thin films and non-thermal plasma exhibit anti-bacterial activity, they together can be more effective in that line and so it is very important to study the effects of CAP on Ag thin films. Specially in food, biology, water, wastewater industries as well as medical science and its instruments [24, 25]. In this work after deposition of silver (thin films) they were exposed to argon and helium cold plasma to investigate their effect on surface morphology, structural characterization as well as studying of their anti-bacterial property on eliminating of E. coli for water treatment.
Experimental details
To produce silver thin films magnetron sputtering method was used. A vacuum system with base pressure of 10−6 m bar was employed (Hind-High-Vacuum, H.H.V., 12″MSPT). For magnetron sputtering argon (purity 99.99%) was used with pressure of 2 × 10−2 mbar. Circular glass with diameter of 2 cm and thickness of 1 mm was employed as substrate. Circular silver disc (purity 99.9%) with thickness of 3 mm and 12.5 cm diameter was made and used as cathode (target for sputtering) and the substrate-to-target distance was fixed at 12 cm (optimum distance after many runs of discharge to obtain an uniform film and suitable coating rate). The deposition rate of 15 Å/S was obtained when plasma discharge current was 0.6 Amp and voltage was at 350 volts. The coating rate and Ag film thickness were measured using a vibrating quartz crystal thickness monitor. During Ag deposition on glass substrate temperature was checked and kept constant using an exact digital thermocouple (namely 300 k). The glass substrates were washed and cleaned in heated acetone ultrasonic bath for 3 min and were dried before deposition of silver.
Before deposition of silver, discharge was working for 2–3 min to remove any possible impurities or contamination on surface of Ag target. A shutter was employed between target and glass substrates and shutter was removed only when line spectrums belonging to Ar and Ag ions and atoms were detected (by spectrometer with high resolving power, via viewing port) and no band spectra due to contamination or impurities was observed. Very pure and clean sputtered silver nanolayer with good uniformity was obtained with 100 and 120 nm thickness. Before and after exposing Ag samples to He and Ar plasma X-ray diffraction (XRD), (X,pert PW3040/60 Philips Holland) with chromium radiation (λcr = 2.289 Å) for Ag films was carried out. To study Ag surface roughness and its variations due to plasma exposure atomic force microscopy (AFM) (Park scientific instrument auto probe model CP) using force constant mode was employed, (scanning area was 5 micron × 5 micron). Antibacterial ability of Ag nanolayer or its particles is good, but at low amount of thickness or concentration this activity is very low [26]. For this reason silver thin films with 100 and 120 nm thickness with good uniformity, high packing density by magnetron sputtering method were produced. For investigation of anti-bacterial activity of the produced silver samples optical density (O.D) method was used, and E. coli (MC 1601 with 106 cfu/ml for colony forming unit, Gram-positive bacteria) was employed. Bacteria and antibacterial reagent were incubated and maintained at 310 k for 24 h. A special fixture was made and Ag samples were suspended in especial cells containing water with bacterium (E. coli) and an exact spectrophotometer with orange light (λ = 600 nm) (Elegant Tech. Cecil series 400, CE 4400) for antibacterial activity measurement was employed. Hydrophilic property for Ag surface was investigated by special camera which could measure contact angle (internal or external) of distribution of pure drop of water on surface of thin films.
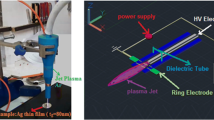
To study the effects of plasma radiation on surface of Ag thin films by Ar and He plasma jet was carried out (see Fig. 1). Ar and He both being inert gases would prevent damaging the Ag thin films from oxidation or carbonation if other gases like (O2, N2, CO and CO2) instead of Ar and He were used. The frequency of 13.56 MHz and power of 15 W was employed, the gas flowing rate of 3 SLM (standard litter per minute) was monitored by a flow meter (Apex-Ac-Ms5, SLPM) and period of 3 min for exposure time for plasma on Ag samples was used. Distance between surface of Ag sample and plasma jet was chosen 10 cm (optimum conditions after many runs) and Ag films were exposed to CAP perpendicular (normal to surface). During plasma treatment its jet temperature was monitored and kept constant (in range of 25–30 °C) using infra-red camera (MSX6).
For plasma jet the first electrode was made of tungsten needle with 1-mm thickness, and the second one was copper ring to serve as the ground electrode. The tungsten electrode was the main electrode which was placed in center of a glass tube with 5 mm inner diameter and plasma (plume) was ejected into surrounding ambient air, Fig. 1 shows schematic set- up of employed plasma jet in this work.
Results and discussion
Structural characterization
To study the influence of non-thermal plasma treatment (He and Ar) on surface of silver thin films, The XRD of produced samples (Ag nanolayers) were carried out. Figures 2 and 3 show XRD pattern of Ag thin films (120 and 100 nm, respectively) before and after being exposed to CAP (using He as plasma gas), No new peak was observed due to plasma treatments which means no impurity or contamination was produced during plasma exposure. Figures 4 and 5 give XRD spectrum of Ag thin films (120 and 100 nm, respectively) before and after argon plasma was used. In both cases the crystalline orientation of peak [111] of Ag was the preferred orientation and plasma radiation did not change the intensity of the peaks very much, and also due to plasma radiation no displacement of the silver peaks was occurred. Average crystalline grain size which was evaluated by Debye–Scherrer’s Eq. (1) was obtained using peak [111] (the most intense peak of Ag) [27, 28].
D is average grain size, λ is X-ray wavelength, B is the full width half at maximum (FWHM), θ is diffraction angle, and k is a dimensionless factor which is taken as 0.95. Crystalline size of Ag thin films before and after being exposed to plasma did not vary that much, for Fig. 2 the average grain size value was changed from 39.5 to 41.2 nm after using He plasma, and for Ag sample in Fig. 3 average crystalline size was 38.4 nm before plasma radiation, and after treatment by Ar CAP became 39.8 nm.
Morphological property
One of the most important parameters in nano-technology and thin films is surface and its roughness, since it is the first interface of material which may interact with other materials and with environment. Surface roughness has vital effects on many phenomena such as sealing, adhesion, friction, and mechanical contact. The most of surfaces are rough in nature and for especial required application specified statistical property is needed. The most commonly used roughness parameters are root mean square (RMS) and average roughness which their values have a sort of positive correlation. The value of RMS roughness somehow is larger than average roughness and rather more sensitive. Root mean square is one of the most important parameters for investigation of rough surfaces and it can be estimated as Eq. (2) [29]:
where R(l,t) is root mean square and \(\bar{h}\) is average surface height and \(h(\overrightarrow {r} ,t)\) is height of a point respect to a reference surface at time t while \(\overrightarrow {r}\) is the vector of point place, \(h(\overrightarrow {r} ,t)\) showing average height of surface of a point for all vector \(\overrightarrow {rs}\). The average height \(\bar{h}\) for a digitized surface is expressed as Eq. (3) [30]:
Often surface roughness increases with film thickness up to a limit (at very high thickness of films or thick films) where we reach saturation deposition after random and correlation parts [30]. Figures 6 and 7 show atomic force microscopy (AFM) 2,3 dimensional (2,3 D) pictures of Ag thin films before (a) and after (b) when helium cold atmospheric plasma was employed. Surface roughness value (RMS) was 3.2 nm before using plasma and after He plasma treatment on surface of Ag thin film (100 nm) its value became 4.3 nm, (see Fig. 6). For silver films (120 nm) using He plasma, fluctuation on surface was more, (see Fig. 7) and RMS roughness increased from 4.2 to 5.8 nm.
Figures 7 and 8 show two- and three-dimensional (2,3 D) AFM images of Ag sample when argon plasma was employed, the RMS roughness was increased from 3.4 to 5.8 nm, after using Ar plasma radiation (Fig. 8 for 100 nm Ag) for Ag thin film (120 nm), RMS roughness increased from 4.3 to 9.1 nm (see Fig. 9) which was much more than He plasma case. From AFM pictures it is clear that surface of Ag films is covered with dense and uniform silver grains. These fluctuations (peaks and valleys) on surface of Ag film produces nanocones which are all grown perpendicular to surface of silver films and enhancement of roughness and nanocones structure mainly are results of radiation pressure from ions, atoms and photons bombardments by plasma treatment.
Antibacterial activity
The rise of surface roughness caused that surface of Ag samples became more hydrophilic which was investigated by special camera for a drop of pure water on Ag films and contact angle (external) was measured which was varied from 85° to 136° after exposure to Ar plasma radiation (see Fig. 10). This property is very important for antibacterial activity of silver nanolayers in removal of E. coli. When Ag surface becomes hydrophilic wider distribution of water on Ag surface was occurred. Since argon and helium (inert gases) for plasma formation were employed surface of the produced samples (silver thin films) did not damaged due to oxidation with other molecular gases (from plasma radiation) which could affect the antibacterial property of Ag films and reduced their activity.
Although the mechanisms behind this activity of nanoscaled Ag on bacteria are not yet fully elucidated, but the most common mechanism behind this activity are proposed to date are uptake of free Ag ions followed by disruption of adenosine triphosphate (ATP) production and deoxyribonucleic acid (DNA) replication and also Ag nanoparticles and its ions generation of reactive oxygen species (ROS) [31].
As it is well known that silver, its thin films and nano particles exhibit antibacterial effect but when cold atmospheric plasma is used on their surfaces this activity become more effective especially when Ar plasma was employed. The exponential growth of E. coli (control) after using Ag sample and silver films after plasma treatment showed reduction of optical density (removal of E. coli) after a short period of lag (the first 2–3 h). This reduction can be due to more surface roughness and higher hydrophilic property of silver thin films in removal of E. coli (see Fig. 11). Penetration of the cell envelope and disorganization of bacterial membrane upon contact with sharp nanocones of silver were indicated to inhibit bacterial growth. Some bacteria such as P. aeruginosa and E. coli are usually resistant microorganism to many standard drugs, or they have low activity against them, but their removal by silver nanocomposites can be helpful [32, 33]. This is due to importance of producing suitable Ag surfaces and employing right method of deposition and coating parameters with help of plasma for surface treatment to obtain better results for water and wastewater treatment.
Conclusion
In this work Ag thin films were exposed to He and Ar plasma radiation and XRD patterns for both cases did not show that much variation after plasma treatment and peak [111] of Ag orientation was the preferred orientation. AFM results revealed that surface of Ag films become rougher after plasma treatment, and this increasing surface roughness is more noticeable when Ar plasma is used. Due to more roughness and hydrophilic property when silver thin films are irradiated by plasma their antibacterial activity were improved and in removal of E. coli these Ag films were more effective when argon plasma was employed. This may be because of particles in Ar plasma (ions, atoms) are heavier than helium and also due to higher radiation pressure by this plasma.
References
Elshabini, A., Elshabini-Riad, A.A., Barlow, F.D.: Thin film technology handbook. McGraw-Hill Professional, London (1998)
Velhal, S.G., Kulkarni, S.D., Latpate, R.V.: Fungal mediated silver nanoparticle synthesis using robust experimental design and its application in cotton fabric. Int Nano Lett 6(4), 257–264 (2016)
Rabor, J.B., Kawamura, K., Muko, D., Kurawaki, J., Niidome, Y.: Spectroscopic properties of triangular silver nanoplates immobilized on polyelectrolyte multilayer-modified glass substrates. Int Nano Lett 7(3), 181–186 (2017)
Dey, A., Dasgupta, A., Kumar, V., Tyagi, A., Verma, A.K.: Evaluation of the of antibacterial efficacy of polyvinylpyrrolidone (PVP) and tri-sodium citrate (TSC) silver nanoparticles. Int Nano Lett 5(4), 223–230 (2015)
Zendehnam, A., Farokhi, B., Beiranvand, N., Miri, S.: Investigation of the statistical surface morphology and optical properties of Ag/Al and Ag/Cu thin double-layers. Int J Thin Sci Tech 3, 181–194 (2013)
Michel, D., Xiao, F., Alameh, K.: A compact, flexible fiber-optic surface plasmon resonance sensor with changeable sensor chips. Sens Actuators B Chem 246, 258–261 (2017)
Shirazi, M., Hosseinnejad, M.T., Zendehnam, A., Ghorannevis, Z., Ghoranneviss, M.: Deposition of ZnO multilayer on LiNbO3 single crystals by DC-magnetron sputtering. Appl Surf Sci 257, 10233–10238 (2011)
Zarrinkhameh, M., Zendehnam, A., Hosseini, S.M.: Fabrication of polyvinylchloride based nanocomposite thin film filled with zinc oxide nanoparticles: morphological, thermal and optical characteristics. J Ind Eng Chem 30, 295–301 (2015)
Zendehdel, M., Zendehnam, A., Hoseini, F., Azarkish, M.: Investigation of removal of chemical oxygen demand (COD) wastewater and antibacterial activity of nanosilver incorporated in poly (acrylamide-co-acrylic acid)/NaY zeolite nanocomposite. Polym Bull 72, 1281–1300 (2015)
Ţălu, Ş., Bramowicz, M., Kulesza, S., Ghaderi, A., Dalouji, V., Solaymani, S., Khalaj, Z.: Microstructure and micromorphology of Cu/Co nanoparticles: surface texture analysis. Electron Mater Lett 12, 580–588 (2016)
Dejam, L., Mohammad Elahi, S., Nazari, H.H., Elahi, H., Solaymani, S., Ghaderi, A.: Structural and optical characterization of ZnO and AZO thin films: the influence of post-annealing. J Mater Sci Mater Electron 27, 685–696 (2016)
Zendehnam, A., Robatmili, N., Hosseini, S.M., Arabzadegan, M., Madaeni, S.S.: Fabrication of novel (acrylonitrile butadiene styrene/activated carbon/silver nanoparticles) heterogeneous anion exchange membrane: physico-chemical and antibacterial characteristics. J Taiwan Inst Chem Eng 44, 670–677 (2013)
Zarrinkhameh, M., Zendehnam, A., Hosseini, S.M.: Preparation and characterization of nanocomposite heterogeneous cation exchange membranes modified by silver nanoparticles. Korean J Chem Eng 31, 1187–1193 (2014)
Dobrynin, D., Fridman, G., Friedman, G., Fridman, A.: Physical and biological mechanisms of direct plasma interaction with living tissue. New J Phys 11, 115020 (2009)
Nam, G., Purushothaman, B., Rangasamy, S., Song, J.M.: Investigating the versatility of multifunctional silver nanoparticles: preparation and inspection of their potential as wound treatment agents. Int Nano Lett 6(1), 51–63 (2016)
Daeschlen, G., Woedtke, T.V., Kindel, E., Denburge, R.B., Junger, M.: Antibacterial activity of an atmospheric pressure plasma jet against relevant wound pathogens in vitro on a simulated wound environment. Plasma Process Polym 7, 224–230 (2010)
Metelmann, H.R., Woedtke, T.V., Bussiahan, R., Weitmann, K.D., Khalili, R.: Experimental recovery of CO2-laser skin lesions by plasma stimulation. Am J Cosmet Surg 29(1), 52–56 (2012)
Mashayekh, A., Akhlaghi, M., Rajaee, H., Khani, M., Shokri, B.: Feasibility of leukemia cancer treatment (K562) by atmospheric pressure plasma jet. J Int Sci 8(10), 723–726 (2014)
Xu, W., Huang, S., Chen, F., Song, J., Liu, X.: Diamond wear properties in cold plasma jet. Diam Relat Mater 48, 96–103 (2014)
Sarani, A., Nikiforov, A.Y., De Geyter, N., Morent, R., Leys, C.: Surface modification of polypropylene with an atmospheric pressure plasma jet sustained in argon and an argon/water vapour mixture. Appl Surf Sci 257, 8737–8741 (2011)
Ramezani, A.H., Sari, A.H., Shokouhy, A.: The effects of argon ion bombardment on the corrosion resistance of tantalum. Int Nano Lett 7(1), 51–57 (2017)
Li, L., Zhang, X., Zhang, M., Li, P., Chu, P.K.: Microporous N-doped carbon film produced by cold atmospheric plasma jet and its cell compatibility. Vacuum 108, 27–34 (2014)
Tadi, A.J., Hosseini, S.R., Semiromi, M.N.: Influence of surface nano/ultrafine structure formed via pre-deep rolling process on the plasma nitriding characteristics of the AISI 316L stainless steel. Int Nano Lett 7(3), 217–223 (2017)
Zendehnam, A., Robatmili, N., Hosseini, S.M., Arabzadegan, M., Madaeni, S.S.: Fabrication of novel (acrylonitrile butadiene styrene/activated carbon/silver nanoparticles) heterogeneous anion exchange membrane: physico-chemical and antibacterial characteristics. J Taiwan Inst Chem Eng 44, 670–677 (2013)
Moshfegh, A.Z.: Nanoparticle catalysts. J Phys D Appl Phys 42, 233001–233065 (2009)
Hedayati, K., Zendehnam, A., Hassanpour, F.: Fabrication and characterization of zinc sulfide nanoparticles and nanocomposites prepared via a simple chemical precipitation method. J Nanostruct 6, 207–212 (2016)
Ţălu, Ş., Solaymani, S., Bramowicz, M., Kulesza, S., Ghaderi, A., Shahpouri, S., Elahi, S.M.: Effect of electric field direction and substrate roughness on three-dimensional self-assembly growth of copper oxide nanowires. J Mater Sci Mater Electron 27, 9272–9277 (2016)
Solaymani, S., et al.: Correlation between the multifractal structure, crystalline and photoluminescence properties of engineered CZO thin films. Int J Hydrog Energy 42, 14205–14219 (2017)
Barbasi, A.L., Stanley, H.E.: fractal concept in surface growth. Cambridge University Press, Cambridge (1995)
Zhao, Y., Wang, G.C., Lu, T.M.: Characterization of amorphous and crystalline rough surface: principles and applications. Exp Methods Phys Sci 37, 354–399 (2000)
Ramos, J.L.: Virulence and gene regulation. Springer, Berlin (2012)
Sedaghat, T., Aminian, M., Bruno, G., Amiri Rudbari, H.: Binuclear organotin (IV) complexes with adipic dihydrazones: synthesis, spectral characterization, crystal structures and antibacterial activity. J Organomet Chem 737, 26–31 (2013)
Marambio-Jones, C., Hoek, E.M.V.: A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanoparticle Res 12, 1531–1551 (2010)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zendehnam, A., Ghasemi, J. & Zendehnam, A. Employing cold atmospheric plasma (Ar, He) on Ag thin film and their influences on surface morphology and anti-bacterial activity of silver films for water treatment. Int Nano Lett 8, 157–164 (2018). https://doi.org/10.1007/s40089-018-0240-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-018-0240-8