Abstract
Background:
Poly(L-lactic acid) (PLLA) is a biodegradable polymer (BP) that replaces conventional petroleum-based polymers. The hydrophobicity of biodegradable PLLA periodontal barrier membrane in wet state can be solved by alloying it with natural polymers. Alloying PLLA with gelatin imparts wet mechanical properties, hydrophilicity, shrinkage, degradability and biocompatibility to the polymeric matrix.
Methods:
To investigate membrane performance in the wet state, PLLA/gelatin membranes were synthesized by varying the gelatin concentration from 0 to 80 wt%. The membrane was prepared by electrospinning.
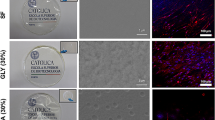
Results:
At the macroscopic scale, PLLA containing gelatin can tune the wet mechanical properties, hydrophilicity, water uptake capacity (WUC), degradability and biocompatibility of PLLA/gelatin membranes. As the gelatin content increased from 0 to 80 wt%, the dry tensile strength of the membranes increased from 6.4 to 38.9 MPa and the dry strain at break decreased from 1.7 to 0.19. PLLA/gelatin membranes with a gelatin content exceeding 40% showed excellent biocompatibility and hydrophilicity. However, dimensional change (37.5% after 7 days of soaking), poor tensile stress in wet state (3.48 MPa) and rapid degradation rate (73.7%) were observed. The highest WUC, hydrophilicity, porosity, suitable mechanical properties and biocompatibility were observed for the PLLA/40% gelatin membrane.
Conclusion:
PLLA/gelatin membranes with gelatin content less than 40% are suitable as barrier membranes for absorbable periodontal tissue regeneration due to their tunable wet mechanical properties, degradability, biocompatibility and lack of dimensional changes.











Similar content being viewed by others
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Castro-Aguirre E, Auras R, Selke S, Rubino M, Marsh T. Insights on the aerobic biodegradation of polymers by analysis of evolved carbon dioxide in simulated composting conditions. Polym Degrad Stab. 2017;137:251–71.
Husarova L, Pekarova S, Stloukal P, Kucharzcyk P, Verney V, Commereuc S, et al. Identification of important abiotic and biotic factors in the biodegradation of poly(L-lactic acid). Intl J Biol Macromol. 2014;71:155–62.
Zaaba NF, Jaafar M. A review on degradation mechanisms of polylactic acid: hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym Eng Sci. 2020;60:2061–75.
Spasova M, Stoilova O, Manolova N, Rashkov I. Preparation of PLLA/PEG nanofibers by electrospinning and potential applications. J Bioact Comp Polym. 2007;22:62–76.
Sitompul JP, Insyani R, Prasetyo D, Prajitno H, Lee HW. Improvement of properties of poly(L-lactic acid) through solution blending of biodegradable polymers. J Eng Technol Sci. 2016;48:430–41.
Solomon S, Sufaru I, Teslaru S, Ghiciuc CM, Stafie CS. Finding the perfect membrane: current knowledge on barrier membranes in regenerative procedures: a descriptive review. Appl Sci. 2022;12:1042.
Sculean A, Nikolidakis D, Schwarz F. Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials–biological foundation and preclinical evidence. J Clin Periodon. 2008;36:106–16.
Martin O, Averous L. Poly(lactic acid): plasticization and properties of biodegradable multiphase systems. Polymer. 2001;42:6209–19.
Badaraev AD, Sidelev DV, Kozelskaya AI, Bolbasov EN, Tran T, Nashchekin AV, et al. Surface modification of electrospun bioresorbable and biostable scaffolds by pulsed DC magnetron sputtering of titanium for gingival tissue regeneration. Polymers (Basel). 2022;14:4922.
Sidelev, DV, Bleykher GA, Bestetti M, Krivobokov VP, Vicenzo, A, Fraz S, et al. A comparative study on the properties of chromium coatings deposited by magnetron sputtering with hot and cooled target. Vacuum. 2017;143;479.
Badaraev AD, Sidelev DV, Yurjev YN, Bukal VR, Tverdokhlebov SI. Modes development of PLGA scaffolds modification by magnetron co-sputtering of Cu and Ti targets. J Phys Conf Ser. 2021;1799;012001.
Sun X, Xu C, Wu G, Ye Q, Wang C. Poly(lactic-co-glycolic acid): Applications and future prospects for periodontal tissue regeneration. Polymers (Basel). 2017;9;189.
Reis ECC, Borges APB, Araujo MVF, Mendes VC, Guan L, Davies JE. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials. 2011;32;9244.
Abdal-hay A, Hussein KH, Casettari L, Khalil KA, Hamdy AS. Fabrication of novel high performance ductile poly(lactic acid) nanofiber scaffold coated with poly(vinyl alcohol) for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2016;60:143–50.
Cho Y, Jeong D, Lee DY. Comparative study on absorbable periodontal tissue regeneration barrier membrane. J Cryst Growth Cryst Technol. 2023;33:71–7.
Cho Y, Jeong H, Kim B, Jang J, Song Y, Lee DY. Electrospun poly(L-lactic acid)/gelatin hybrid polymer as a barrier to periodontal tissue regeneration. Polymers. 2023;15:3844.
Annunziata M, Nastri L, Cecoro G, Guida L. The use of poly-D,L-lactic acid (PDLLA) devices for bone augmentation techniques: a systematic review. Molecules. 201;22;2214.
Yan S, Xiaoqiang L, Shuiping L, Hongsheng W, Chuanglong H. Fabrication and properties of PLLA-gelatin nanofibers by electrospinning. J Appl Polym Sci. 2010;117:542–7.
Shen R, Xu W, Xue Y, Chen L, Ye H, Zhong E, et al. The use of chitosan/PLA nanofibers by emulsion electrospinning for periodontal tissue engineering. Artif Cell Nanomed Biotechnol. 2018;46:419–30.
Lu H, Oh HH, Kawazoe N, Yamgishi K, Chen G. PLLA-collagen and PLLA-gelatin hybrid scaffolds with funnel-like porous structure for skin tissue engineering. Sci Technol Adv Mater. 2012;13:064210.
Casasola R, Thomas NL, Trybala A, Georgiadou S. Electrospun poly lactic acid (PLA) fibres: effect of different solvent systems on fibre morphology and diameter. Polymer. 2014;55:4728–37.
Jeong H, Rho J, Shin J, Lee DY, Hwang T, Kim KJ. Mechanical properties and cytotoxicity of PLA/PCL films. Biomed Eng Lett. 2018;8:267–72.
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Exfahani M, Ramakrishna S. Electrospun poly(ε-caprloactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29:4532–9.
Salehi M, Niyakan M, Ehterami A, Haghi-Daredeh S, Nazarnezhad S, Abbaszadeh-Goudarzi G, et al. Porous electrospun poly(ε-caprloactone)/gelatin nanofibrous mat containing cinnamon for wound healing application, in vitro and in vivo study. Biomed Eng Lett. 2020;10:149–61.
Song Y, Kim B, Yang D, Lee DY. Poly(ε-caprolactone)/gelatin nanofibrous scaffolds for wound dressing. Appl Nanosci. 2022;12:3261–70.
Jeong H, Lee DY, Yang D, Song Y. Mechanical and cell-adhesive properties of gelatin/polyvinyl alcohol hydrogels and their application in wound dressing. Macromol Res. 2022;30:223–9.
Yusof MR, Shamsudin R, Zakaria S, Hamid MAA, Yalcinkaya F, Abdullah Y, et al. Fabrication and characterization of carboxymethyl starch/poly(L-lactide) acid/β-tricalcium phosphate composite nanofibers via electrospinning. Polymers (Basel). 2019;11:1468.
Alberti C, Damps N, Meibner RRR, Hofmann M, Rijono D, Enthaler S. Selective degradation of end-of-life poly(lactide) via alkali-metal-halide catalysis. Adv Sustain Syst. 2019;4:1900081.
Mikhailov OV. Gelatin as it is: history and modernity. Intl J Mol Sci. 2023;24:3583.
Alipal J, Puad NSASM, Lee TC, Nayan NHM, Sahari N, Basri H, et al. A review of gelatin: properties, sources, process, applications, and commercialization. Mater Today Proc. 2021;42:240–50.
He W, Fureh J, Shao J, Gai M, Hu N, He Q. Guidable GNR-Fe3O4-PEM@SiO2 composite particles containing near infrared active nanocalorifiers for laser assisted tissue welding. Colloids Surf A Physicochem Eng Asp. 2016;511:73–81.
Meddahi-Pelle A, Legrand A, Marcellan A, Louedec L, Letourneur D, Leibler L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew Chem Int Ed Engl. 2014;53:6369–73.
Wojak-Cwik IM, Hintze V, Schnabelrauch M, Moeller S, Dobrzynski P, Pamula E, et al. Poly(L-lactide-co-glycolide) scaffolds coated with collagen and glycosaminoglycans: impact on proliferation and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 2013;101:3109–22.
Li W, Zhou J, Xu Y. Study of the in vitro cytotoxicity testing of medical devices (review). Med Rep. 2015;3:617–20.
Khalili AA, Ahmad MR. A review of cell adhesion studies for biomedical and biological applications. Intl J Mol Sci. 2015;16:18149–84.
Shalumon KT, Deepthi S, Anupama MS, Nair SV, Jayakumar R, Chennazhi KP. Fabrication of poly(L-lactic acid)/gelatin composite tubular scaffolds for vascular tissue engineering. Intl J Biol Macromol. 2015;72:1048–55.
Tamayo L, Santana P, Forero JC, Leal M, Gonzalez N, Diaz M, et al. Coaxial fibers of poly(styreneco- maleic anhydride)@poly(vinyl alcohol) for wound dressing applications: Dual and sustained delivery of bioactive agents promoting fibroblast proliferation with reduced cell adherence. Intl J Pharm. 2022;611;121292.
Xu L, Crawford K, Gorman CB. Effect of temperature and pH on the degradation of poly(lactic acid) brushes. Macromolecules. 2011;44:4777–82.
Zulkifli FH, Hussain FSJ, Rasad AMSB, Yusoff MM. In vitro degradation study of novel HEC/PVA/collagen nanofibrous scaffold for skin tissue engineering applications. Polym Degrad Stab. 2014;110:473–81.
Hausberger AG, DeLuca PP. Characterization of biodegradable poly(D, L-lactide-co-glycolide) polymers and microspheres. J Pharm Biomed Anal. 1995;13:747–60.
Lima KM, Rodrigues Junior JM. Poly-DL-lactide-co-glycolide microspheres as a controlled release antigen delivery system. Braz J Med biol Res. 1999;32:171–80.
Capan Y, Woo BH, Gebrekidan S, Ahmed S, DeLuca PP. Preparation and characterization of poly(D,L-lactide-co-glycolide) microspheres for controlled release of poly(L-lysine) complexed plasmid DNA. Pharm Res. 1999;16;509.
Acknowledgements
This research was supported by the Technology Development Program (Project No. S3301367) funded by the Ministry of SMEs and Startups (MSS, Republic of Korea). We would like to thank Editage (www.editage.co.kr) for English language editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Yunyoung Jang, Juwoong Jang, Bae-Yeon Kim, Yo-Seung Song and Deuk Yong Lee declare they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jang, Y., Jang, J., Kim, BY. et al. Effect of Gelatin Content on Degradation Behavior of PLLA/Gelatin Hybrid Membranes. Tissue Eng Regen Med 21, 557–569 (2024). https://doi.org/10.1007/s13770-024-00626-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-024-00626-4