Abstract
Tissue damage caused by various stimuli under certain conditions, such as biological and environmental cues, can actively induce systemic and/or local immune responses. Therefore, understanding the immunological perspective would be critical to not only regulating homeostasis of organs and tissues but also to restrict and remodel their damage. Lungs serve as one of the key immunological organs, and thus, in the present article, we focus on the innate and adaptive immune systems involved in remodeling and engineering lung tissue. Innate immune cells are known to react immediately to damage. Macrophages, one of the most widely studied types of innate immune cells, are known to be involved in tissue damage and remodeling, while type 2 innate lymphoid cells (ILC2s) have recently been revealed as an important cell type responsible for tissue remodeling. On the other hand, adaptive immune cells are also involved in damage control. In particular, resident memory T cells in the lung prevent prolonged disease that causes tissue damage. In this review, we first outlined the structure of the respiratory system with biological and environmental cues and the innate/adaptive immune responses in the lung. It is our hope that understanding an immunological perspective for tissue remodeling and damage control in the lung will be beneficial for stakeholders in this area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The purpose of tissue engineering is to design functional constructs that restore, maintain, or improve damaged tissues or organs in animals and humans [1]. Lung tissue engineering is an emerging field that focuses on developing replacement devices and tissue regeneration. Lung tissue damage may be caused by viral infections, allergens, physical forces, or other forms of stressors. It is critical to reduce tissue damage since it frequently results in disease or/and sometimes immunological pathology that calls for an understanding of the immunological perspective. Therefore, although numerous factors are needed in tissue engineering [2], immune cell involvement and function should be considered. The majority of immune cell research in the field of tissue engineering focuses on macrophages. It is crucial, however, to remember that it is critical to consider other immune cells, not only macrophages, in tissue engineering.
The lung is an important immunological interface. The respiratory tract is exposed to incoming air that contains both harmless ambient components and potentially harmful substances, including allergens and pathogenic airborne microorganisms. Stimuli such as microbe-related substances or tissue damage could actively induce systemic and/or local immune responses. Therefore, to protect the host in such a close contact zone, the respiratory immune system must be fine-tuned and quick to react, with the ability to immediately detect harmful microbes [3, 4]. Defenses in the lung are carried out primarily by immune cells, including alveolar macrophages that are present in the airways and neutrophils that migrate upon receiving alarming signals, such as chemokines [5]. Immune cells respond to foreign substances by releasing various effector molecules, such as chemokines, cytokines, defensins, or mucins [6]. Needless to say, understanding the mechanisms for the protective immune responses that take place within the lung is crucial for tissue engineering.
Early recognition of pathogens with an immediate response by the innate immune system and prompt removal of these pathogens by means of defensive mechanisms demonstrate the effectiveness of innate immune cells in the airway and lung. When innate immune cells such as macrophages and dendritic cells (DCs) recognize pathogens via pathogen recognition receptors (PRRs), they are activated and subsequently produce proinflammatory cytokines. Moreover, innate lymphoid cells (ILCs) are present in various mucosal tissues, including the lung, which serve as early responders to invading pathogens and allergens [7]. Notably, type 2 ILCs are the most prevalent ILCs in the lung [8], as they play important roles in lung tissue homeostasis and remodeling [9]. Innate immunity provides an effective initial defense against infections, although many pathogenic microbes have evolved resistance. In turn, we as hosts have developed adaptive immune responses.
It has been proposed that the increased number of localized infections and injuries brought on by the unique jaw structures and predatory lifestyle of primitive jawed fish (placoderms) led to the evolution of the adaptive immune system of vertebrates [10]. Adaptive immune systems display a high degree of specificity together with remarkable memory cell and antibody secretion properties. There are two types of lymphocytes, T cells and B cells, that mediate adaptive immune responses. T cells can be further divided into CD4+ and CD8+ T cells [11]. In addition to assisting in the activation of CD8+ T cells, CD4+ T cells facilitate the differentiation and maturation of B cells to secrete antibodies and activate macrophages to clear ingested microbes. Effector CD8+ T cells, also called cytotoxic T lymphocytes, can kill infected target cells directly but only after antigen processing and presentation by DCs [12]. It has been suggested that CD4+ and CD8+ T cells play critical roles not only in protection but also in damage control in lung tissue. As a result, maintaining lung tissue requires remodeling and damage control of these tissues via both innate and adaptive immunity, respectively.
In this review, we first provide an overview of the respiratory tract along with various factors that affect tissue damage. We then focus on immune cell traits for lung tissue engineering, specifically ILCs as a component of innate immunity and T cells as a component of adaptive immunity.
2 Respiratory system
Lung tissue engineering requires a thorough understanding of the cellular composition and structural organization of the respiratory system. Furthermore, lungs are vulnerable to biological and environmental cues that sometimes result in wounds and, consequently, diseases. Therefore, understanding these cues is essential for remodeling and regulating lung tissue. In the current section, we shall outline the structure of the respiratory system with biological and environmental cues.
2.1 Anatomical composition
The respiratory tract is a complex organ system. The extensively branched airways in lungs distribute incoming air from the mouth and trachea across their lobes [13]. The upper and lower respiratory tracts are two divisions of the respiratory system. The upper respiratory tract includes the mouth, throat, nose or nostrils, nasal cavity and larynx, which primarily serve as an airway. The lower respiratory tract consists of the trachea and lungs, which are further split into terminal and respiratory airways and peripheral alveoli, where gas exchange takes place.
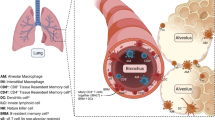
Airways and blood vessels are part of the branching tissue networks that make up the respiratory system. Regional variations in cellular composition reflect the distinct purposes of each location [14]. The respiratory epithelium is made up of numerous unique cell types. The proximal airway (Fig. 1A) is composed of ciliated cells, mucus-producing goblet cells, secretory club cells, undifferentiated basal cells and neuroendocrine cells. On their apical surfaces, ciliated cells have cilia that transport microbes and mucus from the airways after inhalation. Goblet cells secrete mucus to trap inhaled microbes; however, if mucus production is excessive, airways become obstructed. Non-ciliated bronchiolar epithelial cells known as secretory club cells produce a variety of proteins for defense and immunomodulatory purposes [15]. The primary stem cells for the airway epithelia are basal cells, while neurons and neuroendocrine cells work together as sensory cells. The distal airway (Fig. 1B) is composed of alveolar type 1 (AT1) and alveolar type 2 (AT2) epithelial cells [16]. Ninety-five percent of the gas exchange surface area is covered by elongated AT1 cells that are in close contact with the endothelial capillary plexus. To prevent alveolar collapse during respiration, AT2 cells that are cuboidal in shape secrete a pulmonary surfactant, which decreases the surface tension. The lungs contain a variety of immune cells that will be discussed in a later section on immune responses.
Outline the structure and cellular composition of the lung. A depiction of the typical longitudinal section of lung with anatomic location for the proximal and distal airways. Respiratory epithelium consists of many distinct cell types, which serve as a defense barrier. A. The proximal airways are composed of ciliated cells, mucus-producing goblet cells, secretory club cells, tuft cells, undifferentiated basal cells and neuroendocrine cells. B. Alveolar type 1 (AT1) and type 2 (AT2) epithelial cells are make up the distal airways. Resident alveolar macrophages constantly scan the local microenvironment for potentially harmful pathogens
2.2 Biological and environmental cues
Respiratory tracts are in direct contact with the environment, which causes the lung to be more vulnerable than other tissues. Biological and environmental cues, including allergens, bacteria, viruses, dust, and physical injury, can damage the lungs. Exposure to allergens causes allergic inflammation and airway obstruction, which leads to asthma development [17]. In addition, asthma is caused by injury or dust damage to lung tissues. Influenza A virus, respiratory syncytial virus (RSV), and coronaviruses typically infect the respiratory tract [18]. The most prevalent bacterial lung diseases include Streptococcus pneumoniae, Staphylococcus aureus, and Mycobacterium tuberculosis [19]. As a result, viral or bacterial infections in the lung could cause pneumonia and bronchitis. The lungs are damaged and afflicted by diseases caused by biological and environmental stressors followed by inflammatory reactions. While inflammatory responses are required to protect and repair lung tissue, excessive or inadequate inflammatory responses could potentially result in immunological pathology and, if severe, catastrophic damage and chronic disease. Therefore, regulating the immune response is critical for the recovery and regeneration of lungs after damage.
3 Immune responses in the lung
As indicated, the respiratory tract is a major portal for pathogens that cause infection and inflammation. Therefore, the lung serves as a key immunological organ since it has both innate and adaptive immune cells that provide potent immune responses. Table 1 enumerates the fundamental characteristics of the innate and adaptive immune responses. Innate immune cells, including macrophages, DCs, and ILCs, provide the first line of defense against pulmonary infections and are required to coordinate the subsequent adaptive immune response. They also assist with the remodeling and repair of damaged tissue. In regard to adaptive immune cells, their focus is not only on helping innate immune cells for tissue repair but also on removing the source of injury for tissue recovery. Adaptive immune cells are composed of CD4+ T cells, CD8+ T cells and B cells. The following section will describe each of these cells in terms of their properties and functions.
3.1 Innate immune cells
Effector molecules, including cytokines and chemokines produced at the site of infection or damage, recruit various innate immune cells. The earliest and fastest cells that react to damage are neutrophils. Monocytes and DCs are then activated. Innate immune cells produce cytokines to promote inflammation first and enzymes involved in wound healing later. It has been reported that ILC2s also affect lung tissue homeostasis and remodeling. The following section will discuss the roles of macrophages, DCs, and ILC2s in tissue remodeling.
3.1.1 Macrophages and dendritic cells
Macrophages are cells of hematopoietic origin that play a crucial role in innate immune defense and have tissue-specific traits for the maintenance and regulation of organ homeostasis. Macrophages are antigen-presenting cells (APCs) that regulate the differentiation and homeostasis of T cells [20]. It is common knowledge that macrophages can be divided into M1 and M2 types [21]. M1 macrophages have been linked to proinflammatory responses, whereas M2 macrophages have been linked to anti-inflammatory responses. M2 macrophages play a critical role in wound healing and tissue remodeling and possess strong anti-inflammatory properties. Although the M1/M2 paradigm has offered a valuable framework for studying macrophage biology, more specific criteria are needed to identify the diversity and different activation states of macrophages that occur in tissues. In the lung, two different macrophage populations have been identified: alveolar macrophages (AMs) and interstitial macrophages (IMs). The airspace is inhabited by AMs, which develop from the embryonic yolk sac. AMs are highly phagocytic cells that clear particles and pathogens inhaled into the lungs, as they are the primary sentinel cells within the lung [22]. Numerous studies on wound healing have been thoroughly conducted since AMs have anti-inflammatory features [23]. AM-derived cytokines such as transforming growth factor (TGF)-β, tumor necrosis factor (TNF)-α, keratinocyte growth factor, and epidermal growth factor are known to assist in tissue repair and remodeling [24].
IMs reside in interstitial spaces and are known to arise from monocytes [25]. IMs consist of a relatively small population that exhibit important immunoregulatory properties. For instance, interleukin (IL)-10-producing IMs inhibit the maturation and migration of DCs in the lung upon LPS stimulation [26]. Increased IM in the lungs as a result of CpG exposure prevent allergic inflammation by producing IL-10 [27]. Although there has not yet been an active research on tissue remodeling, it is anticipated that regulating IM function will be helpful for tissue remodeling.
DCs, one of the professional APCs, are the only cell type capable of inducing differentiation of naive T cells. DCs are classified as conventional DCs (cDCs), plasmacytoid DCs (pDCs), and monocyte-derived DCs (moDCs). DCs are known to bridge the innate and adaptive immune systems by delivering antigens from the effector site to the draining lymph nodes, where they interact with T and B cells [28]. The cross-presentation, a cDC1 specialty, to CD8+ T cells is essential for protective immunity against intracellular bacteria, viruses, and cancers. In the lung, CD4+ T cell differentiation into subsets with an emphasis on antiviral, antifungal, or antihelminth immunity is promoted by cDC2s, the major producers of proinflammatory chemokines, which are known to be responsible for recruiting inflammatory cells [29]. During respiratory viral infections, cDC2s are activated in a type I interferon (IFN)-dependent manner and prime CD4+ and CD8+ T cells [30]. Although pDCs do not do a good job for presenting antigens, they are a significant early source of type I IFN during viral infection. At the early stage of infection or tissue damage, DCs can induce immunopathology in the lung, causing a fibrotic airway response [31] or chronic obstructive pulmonary disease (COPD) [32]. Thus, understanding the exact role of DCs in the lung is critical for lung tissue disease. Needless to say, targeting the immune regulation of lung DCs may be a valuable strategy to prevent or treat airway tissue remodeling, although more research is needed.
3.1.2 Innate lymphoid cells
ILCs are a novel type of innate immune cell that resembles lymphocytes in both phenotype and function [6]. ILCs are mostly found in mucosal tissues, where they are poised to respond promptly to environmental insults, including pathogen invasions. ILCs do not express antigen-specific receptors but instead are activated by multiple cytokines. Once triggered, ILCs secrete large quantities of cytokines that mediate immune responses, which will be discussed in the following section. ILCs are classified into type 1 (ILC1), type 2 (ILC2), and type 3 (ILC3) innate lymphoid cells and regulatory ILCs (ILCregs) depending on their developmental and effector programs defined by the expressions of lineage-specific transcription factors [33]. ILCs are described as innate counterparts to CD4+ T cells, since mirroring cytokines are released by Th1, Th2, Th17, and Treg cells (Fig. 2). ILC1s, like Th1 cells, utilize T-bet as a transcription factor and produce IFN-γ and TNF-α upon activation by IL-1, IL-12, and IL-15. ILC1s defend against intracellular bacteria and viruses. GATA3 is a transcription factor for ILC2s, such as Th2 cells, which produce IL-5, IL-9, and IL-13 upon activation by IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) [34]. They are important in tissue homeostasis, helminth clearance, and the pathogenesis of allergic diseases. RORγt is the transcription factor for ILC3s, such as Th17 cells, responsible for IL-17 and IL-22 production upon activation by IL-1β and IL-23. ILC3s are known to protect against extracellular microorganisms and ironically promote autoimmune disorders [35]. Recently, ILCregs, which are similar to Treg cells, were reported [36]. ILCregs rely on the transcriptional regulator, Id3, and produce IL-10 and TGF-β. Further studies are needed to determine which cytokines are involved in the activation of ILCregs. As mentioned above, ILC2s are involved in the repair and homeostasis of lung tissue as follows.
Illustration of characteristic comparison between ILC and CD4+ T cell. ILCP differentiates into ILC1, ILC2, or ILC3, while ILCreg is derived directly from CHILP. By contrast, CD4+ T cells can be classified into Th1, Th2, Th17, Treg and Tfh. ILCs are described as innate counterparts to CD4+ T cells. The diagram depicts the overlapping roles of ILCs and CD4+ T cells with their subtypes. To note, ILC2 and Th2 are crucial for tissue homeostatic maintenance and remodeling in the lung. CHILP, common helper innate lymphoid progenitor; ILCP, innate lymphoid cell precursor; TSLP, thymic stromal lymphopoietin; APC, antigen presenting cell
Regarding the fate of ILC2s, IL-25, IL-33, and TSLP are the best known cytokines [33]. TSLP is required for the survival of ILC2s, whereas its activation relies on IL-33, which is greater when combined with IL-2 or TSLP [37], resulting in IL-5, IL-9, IL-13 and amphiregulin secretion. The classic markers, IL-7R and CRTH2, in humans are downregulated [38], while the surface expressions of c-kit increase when ILC2s are activated by IL-33 and TSLP. IL-5 promotes the differentiation, maturation, and activation of eosinophils, a typical cell type in type 2 immunity. IL-9 produced by ILC2s facilitates not only homeostasis of the lung but also tissue repair during the recovery phase after Nippostrongylus brasiliensis-induced lung inflammation [39]. IL-13 produced by ILC2s increased collagen deposition and airway hyperreactivity, resulting in aggravation of tissue damage and asthma symptoms. On the other hand, IL-13 causes accumulations of M2 macrophages, which assist in repairing lung tissue damage [40]. Since there are conflicting research results and debate in relation to IL-13, further studies considering tissue damage associated with certain disease statuses and the combinations of cytokines released are yet to be revealed. Amphiregulin, an epidermal growth factor family, is mainly produced by ILC2s [41] and is essential for efficient wound healing [42]. In response to influenza virus infections, amphiregulin produced by lung ILC2s promotes airway epithelial integrity and lung tissue homeostasis [43].
These findings unequivocally show that innate immune cells, particularly ILC2s, are crucial for lung tissue remodeling. Additionally, lung tissue engineering would be successful when IL-5, IL-9, IL-13 and amphiregulin are considered for tissue remodeling.
3.2 Adaptive immune cells
In addition to eliciting cues to complete recovery, it is crucial to remodel and repair damaged tissue, as described earlier. Thus, one should consider immune cell function when considering tissue repair. Consequently, adaptive immune responses are essential for cleaning up the mess, repairing damage and maintaining homeostasis of healthy tissue. To protect the lungs from pathogens that can survive in macrophages and extracellular organisms that evade phagocytosis, adaptive immune responses are crucial. The adaptive immune responses are provided by CD4+ T, CD8+ T, and B cells.
3.3 CD4+ T cells
When naive CD4+ T cells contact their cognate antigen by APCs, they differentiate into effector cells and then memory cells, which are essential for protective immune responses. Effector CD4+ T cells can be classified into distinct subsets with unique phenotypes and functions. That is, following an antigen encounter by DCs and lineage determination that is governed by a unique cytokine environment (e.g., IL-12, IL-4, TGF-β/IL-6, or TGF-β), naive CD4+ T cells differentiate into helper T (Th)1, Th2, Th17, follicular helper T (Tfh) and regulatory T cells (Treg) (Fig. 2) [12].
Type 2 immunity, induced by CD4+ T cells, is characterized by the production of IL-4, IL-5, IL-9 and IL-13 in tissues after allergic inflammation or helminth parasitic infections [44]. The numerous functions of type 2 immunity to protect the host include management of tissue regeneration, suppression of excessive type 1 inflammation, preservation of barrier defenses and maintenance of metabolic homeostasis.
Activation of peripheral infection-induced CD4+ T cells is essential not only for CD8+ T-cell activity and survival but also for the development of protective CD8+ T cell memory. While antigen peptide-MHC recognition by T cell receptors (TCRs) on naive CD8+ T cells together with co-stimulatory signals from mature DCs are essential for initiating CD8+ T cell responses, other factors, such as CD4+ T cells, have been shown to significantly influence this process [45].
Th1 cells are characterized by the production of their signature cytokines, namely, IL-2, TNF-α and IFN-γ [46]. Differentiation of Th1 cells requires IL-12 and TBX21 as master transcription factors and phosphorylation of the signaling transducer and activator of transcription (STAT)4 [11]. During this process, IFN-γ signals in an autocrine and paracrine manner to enhance Th1 differentiation and lineage commitment. IFN-γ can promote macrophage activation, mediate anti-microbial immunity, and orchestrate activation of the innate immune system. IFN-γ regulates cellular immunity against Mycobacterium tuberculosis, Listeria monocytogenes, Salmonella typhimurium, and fungal infections in the lung [47]. For instance, in a murine model of Coccidioides immitis infections, neutralization of IFN-γ significantly impaired the resistance ability, but treatment of susceptible BALB/c mice with recombinant IFN-γ significantly protected them from systemic exposure [48]. In a murine model of M. tuberculosis infection, Th1 cells could induce activation of infected macrophages by releasing the Th1-specific cytokines, IFN-γ and TNF-α. Given that the infected macrophages produce reactive oxygen species and nitric oxide in this manner [46], those cytokines are crucial mediators for limiting the growth of intra endosomal M. tuberculosis development and killing the bacteria [49].
Th2 cell differentiation, regulated by the master transcription factors, GATA3 and STAT6, requires IL-4. Once activated, Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13, which promote the development, maturation, and class switching of B cells. Pulmonary eosinophilia is a key factor in allergic airway inflammation and possible contributor to the alterations in airway responsiveness [50]. IL-5 induces eosinophil transmigration through the endothelial and epithelial layers into airways and alveoli, and IL-13 has the potential to induce airway hyperresponsiveness, goblet cell metaplasia and mucus hypersecretion. Additionally, it has been reported that macrophages treated with IL-4 and IL-13 are essential for reducing inflammation and restoring tissue homeostasis [51]. Following injury, epithelial cells proliferate and restore barrier functions in response to IL-4 and IL-13 signaling [44]. Notably, both IL-4 and IL-13 play major roles in the differentiation of M2 macrophages [44]. Indeed, these cytokines increase during helminth infections, which supports the notion that they could aid in preventing tissue damage brought on by parasites [44]. Th2 responses are also known to suppress Th1- and Th17-driven inflammation and aid in tissue repair and regeneration after lung damage. By reducing tissue inflammation and activating vital tissue-regenerative pathways, Th2 cells participate in crucial protective activities.
In response to TCR activation, Th17 cells are differentiated from naive CD4+ cells by engaging antigens. Early Th17-cell development relies on key cytokines, including IL-6, TGF-β, and either IL-23 or IL-21. Once activated, Th17 cells express the enhanced IL-23 receptor, which is essential for Th17 maintenance and expansion. Through STAT3 signaling, IL-6 and IL-21 promote the expression of the Th17 transcriptional regulator, RORγT. It is important to note that the Th17 and IL-17 responses in the lung are not always protective [11]. Indeed, greater weight loss and longer recovery periods are associated with the pathological role of IL-17, which increases after influenza infections [52]. On the other hand, IL-17 is necessary for host defenses against extracellular pathogens such as Klebsiella pneumoniae and Mycoplasma pneumoniae [53, 54].
Bcl-6 expressions together with other cell surface markers, such as CXCR5, PD-1 and ICOS, are characteristic of follicular helper T cells (Tfhs). They are prompted to migrate into germinal centers (GCs) because of CXCR5 expression, where they engage with B cells to trigger a class switch. In addition, B cells can further differentiate into memory B cells or long-lived plasma cells that allow long-lasting antibody production. Tfh cells control this process by producing IL-21, which promotes B cell proliferation and by directly co-stimulating the B cells through interaction with the co-stimulatory molecule, CD40. The role of Tfh cells in supporting memory B cells during humoral immunity has been fully outlined in a recent review paper [55].
Immune system activation is well balanced by regulatory T lymphocytes (Tregs), also referred to as suppressor T cells, which uphold the tolerance to self-antigens and prevent autoimmune disease. In other words, Tregs are essential for both maintaining and re-establishing a homeostatic environment as well as for regulating excessive inflammation. Treg depletion increased allergic airway sensitization and eosinophilic airway inflammation in a mouse model [56]. Therefore, even if they are not directly involved in the clearance of pulmonary pathogens, Tregs orchestrate their actions by influencing other CD4+ subsets, CD8+ T cells, and myeloid cells. Importantly, innate cells that exhibit Treg properties have been discovered and are receiving attention as an immunomodulatory therapy. FoxP3, the key immune-repressive transcription factor of Tregs, can be expressed conditionally by macrophages in stroke lesions. This study demonstrated a unique set of FoxP3+ macrophages with enhanced scavenging capability that may be the focus of immunomodulatory therapy for AIS [57].
3.4 CD8+ T cells
In their resting state, naive CD8+ T cells circulate between the blood and secondary lymphoid organs in search of appropriate peptide–MHC complexes [58]. When specialized CD8+ T cells recognize the appropriate viral peptide-MHC complexes in DCs, they become activated (primed), proliferate, and eventually mature into effector cells that can kill infected cells [59]. The CD8+ T cell response, in brief, develops through three distinct stages (Fig. 3): clonal expansion of antigen-specific T cells to produce large numbers of effector cells at approximately 7 days post-infection (dpi); contraction of the majority (90–95%) of effector cells through apoptosis at 15 dpi; and finally, development of a stable memory population from the cells that survived later than 30 dpi [46]. The lungs are included in these systemic immune responses that occur throughout the entire body. In particular, the kinetics of virus-specific CD8+ T cells peaked at approximately 10–14 days for influenza A virus (IAV), human metapneumovirus and pneumonia virus in contrast to approximately 6–8 days for systemic viral infections [60]. During the activation phase, virus-specific CD8+ T cells upregulate the expressions of markers associated with activation, such as CD25, NKG2a, and CD44, while downregulating the lymphoid homing receptor, CD62L [61]. Effector CD8+ T cells release IFN-γ, TNF-α, and IL-2. Most responding CD8+ T cells undergo apoptosis during the contraction phase, which results in a reduction in the overall number of antigen-specific CD8+ T cells and development of a stable memory population. Central memory cells (TCMs) are a subset of the memory CD8+ T cell population, which mostly circulate between the blood and secondary lymphoid organs and express CD62L, CCR7, and the IL-7 receptor α-chain [62].
CD8+ T cell responses following respiratory pathogen infection. Upon the appropriate antigen presentation by APCs, naive CD8+ T cells become activated, as measured by the upregulation of activation markers, such as CD44, and the downregulation of the lymphoid homing receptor, CD62L. Then, frequency and numbers of activated CD8+ T cells expand in the lung. Complete viral clearance from the lung occurs at the same time as the peak of pulmonary CD8+ T cell expansion. After the peak expansion, contraction occurs to reduce the total number of CD8+ T cells followed by forming a memory population. Two memory CD8+ T cell populations are formed as following; TEM (CD62LloCCR7loIL-7RαloKLRG1hi) that predominate within the lung but also capable of circulating, and TRM (CD62LloCD69hiCD103hi) that represent a lung-resident population of memory CD8+ T cells. TEM, Effector memory T cell; TRM, Tissue resident memory T cell
In most cases of respiratory infections, the great majority of virus-specific memory CD8+ T cells are found within the lung parenchyma rather than traveling through the pulmonary vasculature [63]. Most memory CD8+ T cells in the lung parenchyma are composed of effector memory (TEM) and tissue-resident memory (TRM) cells. TRMs are a subset of non-circulating memory CD8+ T cells that are unique to the peripheral organs [64] and can be characterized by their expressions of the classic tissue-residential markers, CD69 and CD103 [65]. The memory CD8+ T cell frequencies in the airway remain elevated during the course of host recovery and for several months following an infection [66]. When compared to the control, the adoptive transfer of memory CD8+ T cells specific for IAV into the airways considerably reduced lung titers following IAV challenges [67]. Similarly, in the presence of airway RSV-specific memory CD8+ T cells, lung viral loads and weight loss were reduced upon subsequent RSV infections [68]. Collectively, tissue-resident memory CD8+ T cells protect the host against secondary respiratory viral infections. In light of the aforementioned, it is important to note that the innate and adaptive immune responses must work together to achieve the removal of harmful biological and environmental cues, maintenance of healthy conditions and repair of damaged tissue.
4 Conclusion
The majority of studies have focused on specific cells, such as macrophages, although some studies have focused on the management and remodeling of damaged tissues. For tissue engineering, it is essential to consider other immune cells in addition to macrophages.
In this review, we focus on the immune cells that should be considered in the remodeling and engineering of lung tissue, especially ILCs and T cells. Among the many innate immune cells, ILC2s could be one of the most significant cells in tissue remodeling. Tissue remodeling can also be regulated by targeting ILC2s; hence, modulating key factors, such as amphiregulin, will be advantageous to tissue engineering. Interestingly, the functions of ILCs appeared to be similar to those of the T helper subsets, Th1, Th2, and Th17. As a result, it is conceivable that innate immune cells directly contribute to tissue repair and remodeling.
Th1 and CD8+ T cells are relatively minor players in tissue repair and remodeling from the perspective of adaptive immune cells, but they play direct roles in successful host defenses against viral infections such as influenza and RSV. Furthermore, preexisting resident memory CD8+ T cells enhance virus clearance and increase survival by preventing prolonged disease. Th2 responses, on the other hand, are known to suppress Th1- and Th17-driven inflammation, which aid in tissue repair and regeneration after tissue damage. Furthermore, CD4+ T cells cause innate immune cells such as M2 macrophages to become active, which indirectly aids in tissue repair. Therefore, understanding these immune cells will be a strategy for genuine tissue recovery since innate and adaptive immune cells must cooperate to recover and rebuild damaged tissue (Fig. 4).
Innate and adaptive immune responses work in concert to eliminate the cues that damage the tissue. To promote optimal immune response against pathogen, the balance between innate and adaptive immunity must be achieved. When it works properly, the innate immune responses not only prevent the spread of pathogens through fast reactions to pathogens but also remodel damaged tissue. They could also initiate the adaptive immune response as the second line of defense. ILC2 among innate immune cells could be one of the most significant cells in tissue remodeling of lung tissue. CD8+ T cells play an important role in host defense against pathogens since they can eliminate the cause of damage. Not only that, Th2 cells among T cells are known to help tissue repair and regeneration. It is important to note that innate and adaptive immune responses must work together for the removal of harmful biological and environmental cues, the maintenance of healthy condition, and the repair of damaged tissue
References
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6.
de Isla N, Huseltein C, Jessel N, Pinzano A, Decot V, Magdalou J, et al. Introduction to tissue engineering and application for cartilage engineering. Biomed Mater Eng. 2010;20:127–33.
Hartl D, Tirouvanziam R, Laval J, Greene CM, Habiel D, Sharma L, et al. Innate immunity of the lung: from basic mechanisms to translational medicine. J Innate Immun. 2018;10:487–501.
Hasenberg M, Stegemann-Koniszewski S, Gunzer M. Cellular immune reactions in the lung. Immunol Rev. 2013;251:189–214.
Harker JA, Lloyd CM. Overlapping and distinct features of viral and allergen immunity in the human lung. Immunity. 2021;54:617–31.
Iwasaki A, Foxman EF, Molony RD. Early local immune defences in the respiratory tract. Nat Rev Immunol. 2017;17:7–20.
Bartemes KR, Kita H. Roles of innate lymphoid cells (ILCs) in allergic diseases: the 10-year anniversary for ILC2s. J Allergy Clin Immunol. 2021;147:1531–47.
Dutton EE, Camelo A, Sleeman M, Herbst R, Carlesso G, Belz GT, et al. Characterisation of innate lymphoid cell populations at different sites in mice with defective T cell immunity. Wellcome Open Res. 2017;2:117.
Kumar V. Innate lymphoid cell and adaptive immune cell cross-talk: a talk meant not to forget. J Leukoc Biol. 2020;108:397–417.
Matsunaga T, Rahman A. What brought the adaptive immune system to vertebrates?—the jaw hypothesis and the seahorse. Immunol Rev. 1998;166:177–86.
Chen K, Kolls JK. T cell-mediated host immune defenses in the lung. Annu Rev Immunol. 2013;31:605–33.
Schmidt ME, Varga SM. Cytokines and CD8 T cell immunity during respiratory syncytial virus infection. Cytokine. 2020;133: 154481.
Zepp JA, Morrisey EE. Cellular crosstalk in the development and regeneration of the respiratory system. Nat Rev Mol Cell Biol. 2019;20:551–66.
Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol. 2021;21:347–62.
Massaro GD, Singh G, Mason R, Plopper CG, Malkinson AM, Gail DB. Biology of the clara cell. Am J Physiol. 1994;266:L101–6.
Hogan B, Tata PR. Cellular organization and biology of the respiratory system. Nat Cell Biol. 2019. https://doi.org/10.1038/s41556-019-0357-7
Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
Clementi N, Ghosh S, De Santis M, Castelli M, Criscuolo E, Zanoni I, et al. Viral respiratory pathogens and lung injury. Clin Microbiol Rev. 2021;34:e00103-20..
Speert DP. Bacterial infections of the lung in normal and immunodeficient patients. Novartis Found Symp. 2006;279:42–51.
Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37.
Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66.
Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16:36–44.
Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30:511–28.
Puttur F, Gregory LG, Lloyd CM. Airway macrophages as the guardians of tissue repair in the lung. Immunol Cell Biol. 2019;97:246–57.
Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93.
Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009;119:3723–38.
Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov S, Fernandes C, et al. Exposure to bacterial CpG DNA protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity. 2017;46:457–73.
Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19:89–103.
Kim TH, Lee HK. Differential roles of lung dendritic cell subsets against respiratory virus infection. Immune Netw. 2014;14:128–37.
Bosteels C, Neyt K, Vanheerswynghels M, van Helden MJ, Sichien D, Debeuf N, et al. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity. 2020;52:1039-56.e9.
Hashimoto M, Yanagisawa H, Minagawa S, Sen D, Goodsell A, Ma R, et al. A critical role for dendritic cells in the evolution of IL-1β-mediated murine airway disease. J Immunol. 2015;194:3962–9.
Freeman CM, Curtis JL. Lung dendritic cells: shaping immune responses throughout chronic obstructive pulmonary disease progression. Am J Respir Cell Mol Biol. 2017;56:152–9.
Panda SK, Colonna M. Innate lymphoid cells in mucosal immunity. Front Immunol. 2019;10:861.
Rodriguez-Rodriguez N, Gogoi M, McKenzie ANJ. Group 2 innate lymphoid cells: team players in regulating asthma. Annu Rev Immunol. 2021;39:167–98.
Melo-Gonzalez F, Hepworth MR. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology. 2017;150:265–75.
Morita H, Kubo T, Rückert B, Ravindran A, Soyka MB, Rinaldi AO, et al. Induction of human regulatory innate lymphoid cells from group 2 innate lymphoid cells by retinoic acid. J Allergy Clin Immunol. 2019;143:2190-201.e9.
Zheng H, Zhang Y, Pan J, Liu N, Qin Y, Qiu L, et al. The role of type 2 innate lymphoid cells in allergic diseases. Front Immunol. 2021;12:586078.
Camelo A, Rosignoli G, Ohne Y, Stewart RA, Overed-Sayer C, Sleeman MA, et al. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv. 2017;1:577–89.
Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–65.
Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, et al. An essential role for Th2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–6.
Zaiss DMW, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–26.
Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–54.
Chen W, Shu Q, Fan J. Neural regulation of interactions between group 2 innate lymphoid cells and pulmonary immune cells. Front Immunol. 2020;11:576929.
Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76.
Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179:8243–51.
Tau GZ, von der Weid T, Lu B, Cowan S, Kvatyuk M, Pernis A, et al. Interferon gamma signaling alters the function of T helper type 1 cells. J Exp Med. 2000;192:977–86.
Kolls JK. CD4(+) T-cell subsets and host defense in the lung. Immunol Rev. 2013;252:156–63.
Magee DM, Cox RA. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun. 1995;63:3514–9.
Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34:807–19.
Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–89.
Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3–11.
Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86:6792–803.
Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27.
Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86.
Olatunde AC, Hale JS, Lamb TJ. Cytokine-skewed Tfh cells: functional consequences for B cell help. Trends Immunol. 2021;42:536–50.
Crother TR, Schröder NW, Karlin J, Chen S, Shimada K, Slepenkin A, et al. Chlamydia pneumoniae infection induced allergic airway sensitization is controlled by regulatory T-cells and plasmacytoid dendritic cells. PLoS One. 2011;6: e20784.
Cai W, Hu M, Li C, Wu R, Lu D, Xie C, et al. FOXP3+ macrophage represses acute ischemic stroke-induced neural inflammation. Autophagy. 2022:1–20.
Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy. 2011;3:1223–33.
Halle S, Halle O, Förster R. Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol. 2017;38:432–43.
Schmidt ME, Varga SM. The CD8 T cell response to respiratory virus infections. Front Immunol. 2018;9:678.
Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166:1813–22.
Ataide MA, Komander K, Knöpper K, Peters AE, Wu H, Eickhoff S, et al. BATF3 programs CD8(+) T cell memory. Nat Immunol. 2020;21:1397–407.
Knudson CJ, Weiss KA, Hartwig SM, Varga SM. The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection. J Virol. 2014;88:9010–6.
Crowl JT, Heeg M, Ferry A, Milner JJ, Omilusik KD, Toma C, et al. Tissue-resident memory CD8(+) T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat Immunol. 2022;23:1121–31.
Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–10.
Heidema J, Lukens MV, van Maren WW, van Dijk ME, Otten HG, van Vught AJ, et al. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol. 2007;179:8410–7.
McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-γ production. J Immunol. 2015;195:203–9.
Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, Tregoning JS. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. 2018;11:290.
Acknowledgements
This work was carried out with partial support from the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ016613 and PJ016201) and the Ministry of Health & Welfare (HV22C0183), Republic of Korea. The current work was also partially supported by the BK21 FOUR Program of the Department of Agricultural Biotechnology, Seoul National University, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical statement
There were neither animal experiments nor clinical investigations in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pyung, Y.J., Park, DJ., Kim, C.G. et al. Remodeling and Restraining Lung Tissue Damage Through the Regulation of Respiratory Immune Responses. Tissue Eng Regen Med 20, 329–339 (2023). https://doi.org/10.1007/s13770-022-00516-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00516-7