Abstract
Background:
Decellularized nerve allografting is one of promising treatment options for nerve defect. As an effort to develop more efficient nerve graft, recently we have developed a new decellularization method for nerve allograft. The aim of this study was to evaluate the effectiveness and biocompatibility of nerve graft decellularized by our newly developed method.
Methods:
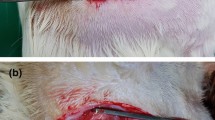
Forty-eight inbred male Lewis rats were divided into two groups, Group I (autograft group, n = 25), Group II (decellularized isograft group, n = 23). Decellularized nerve grafts were prepared with our newly developed methods using amphoteric detergent and nuclease treatment. Serum cytokine level measurements at 0, 2, and 4 weeks and histologic evaluation for inflammatory cell infiltration at 6 and 16 weeks after nerve graft.
Results:
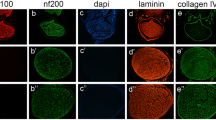
There was no significant difference in mean maximum isometric tetanic force and weight of tibialis anterior muscle or ankle angle at toe-off phase between two groups at 6 and 16 weeks survival time points (p > 0.05). There was no inflammatory cell infiltration in either group and histomorphometric assessments of 6- and 16-week specimens of the isograft group did not differ from those in the autograft group with regard to number of fascicle, cross sectional area, fascicle area ratio, and number of regenerated nerve cells.
Conclusion:
Based on inflammatory reaction, axonal regeneration, and functional outcomes, our newly developed decellularized nerve grafts were fairly biocompatible and had comparable effectiveness to autografts for nerve regeneration, which suggested it would be suitable for nerve reconstruction as an alternative to autograft.





Similar content being viewed by others
References
Safa B, Jain S, Desai MJ, Greenberg JA, Niacaris TR, Nydick JA, et al. Peripheral nerve repair throughout the body with processed nerve allografts: Results from a large multicenter study. Microsurgery. 2020;40:527–37.
Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–52.
Walsh S, Biernaskie J, Kemp SW, Midha R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience. 2009;164:1097–107.
Kim JK, Koh YD, Kim JO, Seo DH. Development of a decellularization method to produce nerve allografts using less invasive detergents and hyper/hypotonic solutions. J Plast Reconstr Aesthet Surg. 2016;69:1690–6.
Waitayawinyu T, Parisi DM, Miller B, Luria S, Morton HJ, Chin SH, et al. A comparison of polyglycolic acid versus type 1 collagen bioabsorbable nerve conduits in a rat model: an alternative to autografting. J Hand Surg Am. 2007;32:1521–9.
Carriel V, Alaminos M, Garzón I, Campos A, Cornelissen M. Tissue engineering of the peripheral nervous system. Expert Rev Neurother. 2014;14:301–18.
Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res Int. 2014;2014:698256.
Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787–99.
Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1–14.
Cho MS, Rinker BD, Weber RV, Chao JD, Ingari JV, Brooks D, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am. 2012;37:2340–9.
Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y). 2009;4:245–9.
Tang P, Kilic A, Konopka G, Regalbuto R, Akelina Y, Gardner T. Histologic and functional outcomes of nerve defects treated with acellular allograft versus cabled autograft in a rat model. Microsurgery. 2013;33:460–7.
Shin RH, Vathana T, Giessler GA, Friedrich PF, Bishop AT, Shin AY. Isometric tetanic force measurement method of the tibialis anterior in the rat. Microsurgery. 2008;28:452–7.
Shin YH, Park SY, Kim JK. Comparison of systematically combined detergent and nuclease-based decellularization methods for acellular nerve graft: an ex vivo characterization and in vivo evaluation. J Tissue Eng Regen Med. 2019;13:1241–52.
Lee JY, Giusti G, Wang H, Friedrich PF, Bishop AT, Shin AY. Functional evaluation in the rat sciatic nerve defect model: a comparison of the sciatic functional index, ankle angles, and isometric tetanic force. Plast Reconstr Surg. 2013;132:1173–80.
Evans PJ, Mackinnon SE, Midha R, Wade JA, Hunter DA, Nakao Y, et al. Regeneration across cold preserved peripheral nerve allografts. Microsurgery. 1999;19:115–27.
Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–29.
Hirasawa Y, Tamai K, Katsumi Y, Sakakida K. Experimental study of nerve allografts: especially on the influence of histocompatibility in fresh nerve grafting. Transplant Proc. 1984;16:1694–9.
Fox IK, Jaramillo A, Hunter DA, Rickman SR, Mohanakumar T, Mackinnon SE. Prolonged cold-preservation of nerve allografts. Muscle Nerve. 2005;31:59–69.
Strasberg SR, Mackinnon SE, Hare GM, Narini PP, Hertl C, Hay JB. Reduction in peripheral nerve allograft antigenicity with warm and cold temperature preservation. Plast Reconstr Surg. 1996;97:152–60.
Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54.
Rovak JM, Bishop DK, Boxer LK, Wood SC, Mungara AK, Cederna PS. Peripheral nerve transplantation: the role of chemical acellularization in eliminating allograft antigenicity. J Reconstr Microsurg. 2005;21:207–13.
Urbanchek MS, Chung KC, Asato H, Washington LN, Kuzon WM Jr. Rat walking tracks do not reflect maximal muscle force capacity. J Reconstr Microsurg. 1999;15:143–9.
Giusti G, Willems WF, Friedrich PF, Jensen MR, Bishop AT, Shin AY. Return of motor function after segmental nerve loss in a rat model: a comparison of autogenous nerve graft, collagen conduit and processed nerve allograft (Axogen). J Bone Joint Surg Am. 2009;7:27–8.
Tang P, Whiteman DR, Voigt C, Miller MC, Kim H. No difference in outcomes detected between decellular nerve allograft and cable autograft in rat sciatic nerve defects. J Bone Joint Surg. 2019;101:e42.
Acknowledgements
This research was supported by a grant of the Korea Health Technology Research and Development Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant No: HI17C1221), and by Seoul St. Mary’s Clinical Medicine Research Program of year 2018 through the The Catholic University of Korea, Seoul, Republic of Korea. This research was supported by a grant (Grant No.: HI17C1221) of the Korea Health Technology Research and Development Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Ethical statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in University (IACUC approval No. CUMC-2019-0108-04).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, D.H., Shin, SH., Lee, MK. et al. Effectiveness and Biocompatibility of Decellularized Nerve Graft Using an In Vivo Rat Sciatic Nerve Model. Tissue Eng Regen Med 18, 797–805 (2021). https://doi.org/10.1007/s13770-021-00353-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00353-0