Abstract
Collagen fibres play an important role in the mechanical behaviour of many soft tissues. Modelling of such tissues now often incorporates a collagen fibre distribution. However, the availability of accurate structural data has so far lagged behind the progress of anisotropic constitutive modelling. Here, an automated process is developed to identify the orientation of collagen fibres using inexpensive and relatively simple techniques. The method uses established histological techniques and an algorithm implemented in the MATLAB image processing toolbox. It takes an average of 15 s to evaluate one image, compared to several hours if assessed visually. The technique was applied to histological sections of human skin with different Langer line orientations and a definite correlation between the orientation of Langer lines and the preferred orientation of collagen fibres in the dermis \((p<0.001, R^{2}= 0.95)\) was observed. The structural parameters of the Gasser–Ogden–Holzapfel (GOH) model were all successfully evaluated. The mean dispersion factor for the dermis was \(\kappa = 0.1404 \pm 0.0028.\) The constitutive parameters μ, k 1 and k 2 were evaluated through physically-based, least squares curve-fitting of experimental test data. The values found for μ, k 1 and k 2 were 0.2014 MPa, 243.6 and 0.1327, respectively. Finally, the above model was implemented in ABAQUS/Standard and a finite element (FE) computation was performed of uniaxial extension tests on human skin. It is expected that the results of this study will assist those wishing to model skin, and that the algorithm described will be of benefit to those who wish to evaluate the collagen dispersion of other soft tissues.













Similar content being viewed by others
Notes
Out-of-plane fibres manifest themselves as circular areas. This condition was introduced to exclude out-of-plane fibres from the analysis. A sensitivity analysis was carried out to determine the sensitivity of the algorithm to varying the area and eccentricity criteria. It was found that a ±10% change in both the area and eccentricity criteria led to a ±1% change in the mean orientation. This indicates that the selected criteria do not significantly affect the result of mean orientation. For this study it was specified that in-plane fibres must have an eccentricity larger than 0.7 and an area greater than 1000 pixels, which for our images, captured at 5×, corresponds to 5 \(\mu \hbox{m}^{2}.\)
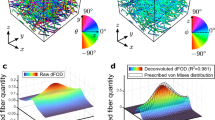
It should be noted here that these results have been obtained using the mle method described in “Fibre Dispersion” section. An alternative, but less reliable method exists whereby data is clustered into two intersecting fibre families using an agglomerative clustering algorithm such as was performed in Ní Annaidh et al.20
References
Bischoff, J. E., E. M. Arruda, and K. Grosh. Finite element modeling of human skin using an isotropic, nonlinear elastic constitutive model. J. Biomech. 33:645–652, 2000.
Bischoff, J. E., E. M. Arruda, and K. Grosh. A rheological network model for the continuum anisotropic and viscoelastic behaviour of soft tissue. Biomech. Model. Mechanobiol. 3:56–65, 2004.
Cortes, D. H., S. P. Lake, J. A. Kadlowec, and L. J. Soslowsky, and D. M. Elliott. Characterizing the mechanical contribution of fiber angular distribution in connective tissue: comparison of two modeling approaches. Biomech. Model. Mechanobiol. 9:651–658, 2010.
Elbischger, P., H. Bischof, P. Regitnig, and G. Holzapfel. Automatic analysis of collagen fiber orientation in the outermost layer of human arteries. Pattern Anal. Appl. 7:269–284, 2004.
Evans, S. L. On the implementation of a wrinkling, hyperelastic membrane model for skin and other materials. Comput. Meth. Biomech. Biomed. Eng. 12:319–332, 2009.
Flamini, V., C. Kerskens, K. M. Moerman, C. K. Simms, and C. Lally. Imaging arterial fibres using diffusion tensor imaging—feasability study and preliminary results. EURASIP J. Adv. Signal. Process. 2010.
Flynn, C., and A. Taberner, P. Modeling the mechanical response of in vivo human skin under a rich set of deformations. Ann. Biomed. Eng. 39:1935–1946, 2011.
Gamage, T. P., V. Rajagopal, M. Ehrgott, M. P. Nash, and P. M. F. Nielsen. Identification of mechanical properties of heterogeneous soft bodies using gravity loading. Int. J. Numer. Methods Biomed. Eng. 27:391–407, 2011.
Gasser, T., R. W. Ogden, and G. Holzapfel. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 3:15–35, 2006.
Haralick, R. M., and L. G. Shapiro. Computer and Robot Vision, vol. 1. Boston: Addison-Wesley, 1992.
Holzapfel, G. A. Handbook of Materials Behavior Models: Biomechanics of Soft Tissue, edited by J. Lemaitre. Academic Press, 2001, pp. 1057–1071.
Holzapfel, G. A. Determination of material models for arterial walls from uniaxial extension tests and histological structure. J. Theor. Biol. 238:290–302, 2006.
Holzapfel, G. A., and R. W. Ogden. On planar biaxial tests for anisotropic nonlinearly elastic solids: a continuum mechanical framework. Math. Mech. Solids 14:474–489, 2009.
Jones, T. A. MATLAB functions to analyze directional (azimuthal) data–I: single-sample inference. Comput. Geosci. 32:166–175, 2006.
Jor, J. W. Y., P. M. F. Nielsen, M. P. Nash, and P. J. Hunter. Modelling collagen fibre orientation in porcine skin based upon confocal laser scanning microscopy. Skin Res. Technol. 17:149–159, 2011.
Jor, J. W. Y., M. P. Nash, P. M. F. Nielsen, and P. J. Hunter. Estimating material parameters of a structurally based constitutive relation for skin mechanics. Biomech. Model. Mechanobiol. 10:767–778, 2011.
Langer, K. On the anatomy and physiology of the skin. The Imperial Academy of Science, Vienna (1861). Reprinted in (1978): Br. J. Plast. Surg. 17:93–106, 1978.
Lanir, Y. Constitutive equations for fibrous connective tissues. J. Biomech. 16:1–12, 1983.
Lanir, Y., O. Lichtenstein, and O. Imanuel Optimal design of biaxial tests for structural material characterization of flat tissues. J. Biomech. Eng. 118:41–46, 1996.
Ní Annaidh, A., K. Bruyère, M. Destrade, M. Gilchrist, and M. Otténio. Characterising the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. Mater. 5:139–148, 2012.
Noorlander, M. L., P. Melis, A. Jonker, and C. J. Van Noorden. A quantitative method to determine the orientation of collagen fibers in the dermis. J. Histochem. Cytochem. 50:1469–1474, 2002.
Ogden, R. W., G. Saccomandi, and I. Sgura. Fitting hyperelastic models to experimental data. Comput. Mech. 34:484–502, 2004.
Otsu, N. A threshold selection method from grey-level histograms. IEEE Trans. Syst. Man Cybern. 9:62–66, 1979.
Ridge, M., and V. Wright. Mechanical properties of skin: a bioengineering study of skin structure. J. Appl. Physiol. 21:1602–1606, 1966.
Ruvolo, E. C., Jr., G. N. Stamatas, and N. Kollias. Skin viscoelasticity displays site- and age-dependent angular anisotropy. Skin Pharmacol. Physiol. 20:313–321, 2007.
Van Zuijlen, P. P. M., H. J. de Vries, E. N. Lamme, J. E. Coppens, J. Van Marle, R. W. Kries, and E. Middelkoop. Morphometry of dermal collagen orientation by Fourier analysis is superior to multi-observer assessment. J. Pathol. 198:284–291, 2002.
Verhaegen, P. D. H. M., E. M. Res, A. Van Engelen, E. Middelkoop, and P. P. M. Van Zuijlen. A reliable, non-invasive measurement tool for anisotropy in normal skin and scar tissue. Skin Res. Technol. 16(3):325–331, 2010.
Wu, J., B. Ragwa, D. Filmer, C. Hoffmann, B. Yuan, C. Chiang, J. Sturgis, and J. Robison. Automated quantification and reconstruction of collagen matrix from 3D confocal datasets. J. Microscopy 210:158–165, 2003.
Yasui, T., Y. Tohno, and T. Araki. Characterization of collagen orientation in human dermis by two-dimensional second-harmonic-generation polarimetry. J. Biomed. Opt. 9:259–264, 2004.
Acknowledgments
The authors acknowledge gratefully the advice and assistance of Mr. Ciaran Driver, Dr. Michael Curtis and Prof. Marie Cassidy, of the Office of the State Pathologist (Ireland), in the area of histology. This research was supported by a Marie Curie Intra European Fellowship within the 7th European Community Framework Programme, awarded to MD; by the Irish Research Council for Science, Engineering and Technology; by the Office of the State Pathologist (Irish Department of Justice and Equality); and by the Ile-de-France region. G.S. is supported by the PRIN 2009 project “Matematica e meccanica dei sistemi biologici e dei tessuti molli”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Ní Annaidh, A., Bruyère, K., Destrade, M. et al. Automated Estimation of Collagen Fibre Dispersion in the Dermis and its Contribution to the Anisotropic Behaviour of Skin. Ann Biomed Eng 40, 1666–1678 (2012). https://doi.org/10.1007/s10439-012-0542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-012-0542-3