Abstract
Background:
Glucosamine hydrochloride (GlcN·HCl) has been shown to inhibit cell growth and matrix synthesis, but not with N-acetyl-glucosamine (GlcNAc) supplementation. This effect might be related to an inhibition of critical growth factors (GF), or to a different metabolization of the two glucosamine derivatives. The aim of the present study was to evaluate the synergy between GlcN·HCl, GlcNAc, and GF on proliferation and cartilage matrix synthesis.
Method:
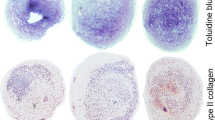
Bovine chondrocytes were cultivated in monolayers for 48 h and in three-dimensional (3D) chitosan scaffolds for 30 days in perfusion bioreactors. Serum-free (SF) medium was supplemented with either growth factors (GF) TGF-β (5 ng mL−1) and IGF-I (10 ng mL−1), GlcN·HCl or GlcNAc at 1mM each or both. Six groups were compared according to medium supplementation: (a) SF control; (b) SF + GlcN·HCl; (c) SF + GlcNAc; (d) SF + GF; (e) SF + GF + GlcN·HCl; and (f) SF + GF + GlcNAc. Cell proliferation, proteoglycan, collagen I (COL1), and collagen II (COL2) synthesis were evaluated.
Results:
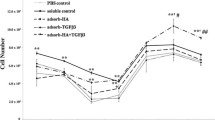
The two glucosamines showed opposite effects in monolayer culture: GlcN·HCl significantly reduced proliferation and GlcNAc significantly augmented cellular metabolism. In the 30 days 3D culture, the GlcN·HCl added to GF stimulated cell proliferation more than when compared to GF only, but the proteoglycan synthesis was smaller than GF. However, GlcNAc added to GF improved the cell proliferation and proteoglycan synthesis more than when compared to GF and GF/GlcN·HCl. The synthesis of COL1 and COL2 was observed in all groups containing GF.
Conclusion:
GlcN·HCl and GlcNAc increased cell growth and stimulated COL2 synthesis in long-time 3D culture. However, only GlcNAc added to GF improved proteoglycan synthesis.







Similar content being viewed by others
References
Scotto d’Abusco A, Politi L, Giordano C, Scandurra R. A peptidyl-glucosamine derivative affects IKKalpha kinase activity in human chondrocytes. Arthritis Res Ther. 2010;12:R18.
Imagawa K, de Andrés MC, Hashimoto K, Pitt D, Itoi E, Goldring MB, et al. The epigenetic effect of glucosamine and a nuclear factor-kappa B (NF-kB) inhibitor on primary human chondrocytes—implications for osteoarthritis. Biochem Biophys Res Commun. 2011;405:362–7.
Chan PS, Caron JP, Rosa GJ, Orth MW. Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E2 in articular cartilage explants. Osteoarthritis Cartilage. 2005;13:387–94.
Ratcliffe A, Hardingham T. Cartilage proteoglycan binding region and link protein. Radioimmunoassays and the detection of masked determinants in aggregates. Biochem J. 1983;213:371–8.
Fenton JI, Chlebek-Brown KA, Peters TL, Caron JP, Orth MW. The effects of glucosamine derivatives on equine articular cartilage degradation in explant culture. Osteoarthritis Cartilage. 2000;8:444–51.
Sandy JD, Gamett D, Thompson V, Verscharen C. Chondrocyte-mediated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem J. 1998;335:59–66.
Jeon JH, Suh HN, Kim MO, Han HJ. Glucosamine-induced reduction of integrin β4 and plectin complex stimulates migration and proliferation in mouse embryonic stem cells. Stem Cells Dev. 2013;22:2975–89.
Bassleer C, Rovati L, Franchimont P. Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic articular cartilage in vitro. Osteoarthritis Cartilage. 1998;6:427–34.
Wang L, Detamore MS. Effects of growth factors and glucosamine on porcine mandibular condylar cartilage cells and hyaline cartilage cells for tissue engineering applications. Arch Oral Biol. 2009;54:1–5.
Dodge GR, Jimenez SA. Glucosamine sulfate modulates the levels of aggrecan and matrix metalloproteinase-3 synthesized by cultured human osteoarthritis articular chondrocytes. Osteoarthritis Cartilage. 2003;11:424–32.
Igarashi M, Kaga I, Takamori Y, Sakamoto K, Miyazawa K, Nagaoka I. Effects of glucosamine derivatives and uronic acids on the production of glycosaminoglycans by human synovial cells and chondrocytes. Int J Mol Med. 2011;27:821–7.
Uitterlinden EJ, Jahr H, Koevoet JL, Jenniskens YM, Bierma-Zeinstra SM, Degroot J, et al. Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthritis Cartilage. 2006;14:250–7.
Varghese S, Theprungsirikul P, Sahani S, Hwang N, Yarema KJ, Elisseeff JH. Glucosamine modulates chondrocyte proliferation, matrix synthesis, and gene expression. Osteoarthritis Cartilage. 2007;15:59–68.
Windhaber RA, Wilkins RJ, Meredith D. Functional characterisation of glucose transport in bovine articular chondrocytes. Pflugers Arch. 2003;446:572–7.
Mobasheri A, Vannucci SJ, Bondy CA, Carter SD, Innes JF, Arteaga MF, et al. Glucose transport and metabolism in chondrocytes: a key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histol Histopathol. 2002;17:1239–67.
Huang JB, Clark AJ, Petty HR. The hexosamine biosynthesis pathway negatively regulates IL-2 production by Jurkat T cells. Cell Immunol. 2007;245:1–6.
Chen YJ, Yao CC, Huang CH, Chang HH, Young TH. Hexosamine-induced TGF-β signaling and osteogenic differentiation of dental pulp stem cells are dependent on N-acetylglucosaminyltransferase V. Biomed Res Int. 2015;2015:924397.
Ali AA, Lewis SM, Badgley HL, Allaben WT, Leakey JE. Oral glucosamine increases expression of transforming growth factor β1 (TGFβ1) and connective tissue growth factor (CTGF) mRNA in rat cartilage and kidney: implications for human efficacy and toxicity. Arch Biochem Biophys. 2011;510:11–8.
Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22.
Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35:547–55.
Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, et al. Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem. 2012;287:35544–55.
Grimaud E, Heymann D, Rédini F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002;13:241–57.
Mariani E, Pulsatelli L, Facchini A. Signaling pathways in cartilage repair. Int J Mol Sci. 2014;15:8667–98.
Miyazaki Y, Tsukazaki T, Hirota Y, Yonekura A, Osaki M, Shindo H, et al. Dexamethasone inhibition of TGF beta-induced cell growth and type II collagen mRNA expression through ERK-integrated AP-1 activity in cultured rat articular chondrocytes. Osteoarthritis Cartilage. 2000;8:378–85.
Li C, Wang Q, Wang JF. Transforming growth factor-β (TGF-β) induces the expression of chondrogenesis-related genes through TGF-β receptor II (TGFRII)-AKT-mTOR signaling in primary cultured mouse precartilaginous stem cells. Biochem Biophys Res Commun. 2014;450:646–51.
Li J, Zhao Z, Liu J, Huang N, Long D, Wang J, et al. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-β1/Smads pathway. Cell Prolif. 2010;43:333–43.
Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, et al. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. J Bone Miner Res. 2006;21:626–36.
Tahimic CG, Wang Y, Bikle DD. Anabolic effects of IGF-1 signaling on the skeleton. Front Endocrinol (Lausanne). 2013;4:6.
Starkman BG, Cravero JD, Delcarlo M, Loeser RF. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem J. 2005;389:723–9.
Zhang M, Zhou Q, Liang QQ, Li CG, Holz JD, Tang D, et al. IGF-1 regulation of type II collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways. Osteoarthritis Cartilage. 2009;17:100–6.
Sun C, Shang J, Yao Y, Yin X, Liu M, Liu H, et al. O-Glc NA cylation: a bridge between glucose and cell differentiation. J Cell Mol Med. 2016;20:769–81.
Lee GM, Tioran ME, Jansen M, Graff RD, Kelley SS, Lin P. Development of selective tolerance to interleukin-1β by human chondrocytes in vitro. J Cell Physiol. 2002;192:113–24.
Tsai TL, Manner PA, Li WJ. Regulation of mesenchymal stem cell chondrogenesis by glucose through protein kinase C/transforming growth factor signaling. Osteoarthritis Cartilage. 2013;21:368–76.
Dehne T, Karlsson C, Ringe J, Sittinger M, Lindahl A. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res Ther. 2009;11:R133.
Pombo-Suarez M, Castaño-Oreja MT, Calaza M, Gomez-Reino J, Gonzalez A. Differential upregulation of the three transforming growth factor beta isoforms in human osteoarthritic cartilage. Ann Rheum Dis. 2009;68:568–71.
Yasuda Y, Nakamura J, Hamada Y, Nakayama M, Chaya S, Naruse K, et al. Role of PKC and TGF-β receptor in glucose-induced proliferation of smooth muscle cells. Biochem Biophys Res Commun. 2001;281:71–7.
Klein AL, Berkaw MN, Buse MG, Ball LE. O-Linked N-acetylglucosamine modification of insulin receptor substrate-1 occurs in close proximity to multiple SH2 domain binding motifs. Mol Cell Proteomics. 2009;8:2733–45.
Song KH, Kang JH, Woo JK, Nam JS, Min HY, Lee HY, et al. The novel IGF-IR/Akt-dependent anticancer activities of glucosamine. BMC Cancer. 2014;14:31.
Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2002;99:5313–8.
de Mattei M, Pellati A, Pasello M, de Terlizzi F, Massari L, Gemmati D, et al. High doses of glucosamine-HCl have detrimental effects on bovine articular cartilage explants cultured in vitro. Osteoarthritis Cartilage. 2002;10:816–25.
Nakatani S, Mano H, Im R, Shimizu J, Wada M. Glucosamine regulates differentiation of a chondrogenic cell line, ATDC5. Biol Pharm Bull. 2007;30:433–8.
Uitterlinden EJ, Jahr H, Koevoet JL, Bierma-Zeinstra SM, Verhaar JA, Weinans H, et al. Glucosamine reduces anabolic as well as catabolic processes in bovine chondrocytes cultured in alginate. Osteoarthritis Cartilage. 2007;15:1267–74.
Shikhman AR, Brinson DC, Valbracht J, Lotz MK. Differential metabolic effects of glucosamine and N-acetylglucosamine in human articular chondrocytes. Osteoarthritis Cartilage. 2009;17:1022–8.
Uldry M, Ibberson M, Hosokawa M, Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002;524:199–203.
Hoffmann B, Seitz D, Mencke A, Kokott A, Ziegler G. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2009;20:1495–503.
Persiani S, Rotini R, Trisolino G, Rovati LC, Locatelli M, Paganini D, et al. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthritis Cartilage. 2007;15:764–72.
Mroz PJ, Silbert JE. Use of 3H-glucosamine and 35S-sulfate with cultured human chondrocytes to determine the effect of glucosamine concentration on formation of chondroitin sulfate. Arthritis Rheum. 2004;50:3574–9.
Henrotin Y, Chevalier X, Herrero-Beaumont G, McAlindon T, Mobasheri A, Pavelka K, et al. Physiological effects of oral glucosamine on joint health: current status and consensus on future research priorities. BMC Res Notes. 2013;6:115.
Miwa I, Mita Y, Murata T, Okuda J, Sugiura M, Hamada Y, et al. Utility of 3-O-methyl-N-acetyl-D-glucosamine, an N-acetylglucosamine kinase inhibitor, for accurate assay of glucokinase in pancreatic islets and liver. Enzyme Protein. 1994–1995;48:135–42.
Hinderlich S, Berger M, Schwarzkopf M, Effertz K, Reutter W. Molecular cloning and characterization of murine and human N-acetylglucosamine kinase. Eur J Biochem. 2000;267:3301–8.
Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–46.
Terry DE, Rees-Milton K, Pruss C, Hopwood J, Carran J, Anastassiades TP. Modulation of articular chondrocyte proliferation and anionic glycoconjugate synthesis by glucosamine (GlcN), N-acetyl GlcN (GlcNAc) GlcN sulfate salt (GlcN.S) and covalent glucosamine sulfates (GlcN-SO4). Osteoarthritis Cartilage. 2007;15:946–56.
Stoppoloni D, Politi L, Leopizzi M, Gaetani S, Guazzo R, Basciani S, et al. Effect of glucosamine and its peptidyl-derivative on the production of extracellular matrix components by human primary chondrocytes. Osteoarthritis Cartilage. 2015;23:103–13.
Acknowledgement
This study was supported by grants from CAPES Foundation within the Ministry of Education of Brazil, University of Bayreuth, and the Friedrich-Baur Biomed Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Ethical statement
There are no animal experiments carried out for this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1.
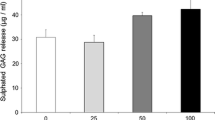
The effect of GlcN·HCl and GlcNAc doses on cell proliferation and viability. A dsDNA quantification (F statistic p < 0.001). * = p < 0.05, compared to 0.5 mM. ** = p < 0.001, compared to 1 mM and 10 mM. # = p < 0.05, compared to 0 mM. B Effect of GlcN·HCl (F statistic, p = 0.24) and GlcNAc (F statistic, p = 0.69) on NADPH/NADH2 synthesis. C Lactate synthesis, (F statistic p < 0.001). * = p < 0.01 compared to 0, 1 µM, and 0.5 mM. # = p < 0.05 compared to 5 mM. § = p < 0.05 compared to control, 1 µM, and 0.5 mM. ƚ = p < 0.001 compared to 5 mM and 10 mM. (PPTX 57 kb)
Rights and permissions
About this article
Cite this article
Pizzolatti, A.L.A., Gaudig, F., Seitz, D. et al. Glucosamine Hydrochloride and N-Acetylglucosamine Influence the Response of Bovine Chondrocytes to TGF-β3 and IGF in Monolayer and Three-Dimensional Tissue Culture. Tissue Eng Regen Med 15, 781–791 (2018). https://doi.org/10.1007/s13770-018-0150-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-018-0150-x