Abstract
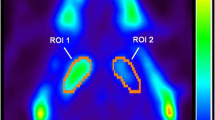
Stem cell technologies are particularly attractive in Parkinson’s disease (PD) research although they occasionally need long-term treatment for anti-parkinsonian activity. Unfortunately, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) widely used as a model for PD has several limitations, including the risk of dose-dependent mortality and the difficulty of maintenance of PD symptoms during the whole experiment period. Therefore, we tested if our novel MPTP regimen protocol (2 mg/kg for 2 consecutive days and 1 mg/kg for next 3 consecutive days) can be maintained stable parkinsonism without mortality for long-term stem cell therapy. For this, we used small-bodied common marmoset monkeys (Callithrix jacchus) among several nonhuman primates showing high anatomical, functional, and behavioral similarities to humans. Along with no mortality, the behavioral changes involved in PD symptoms were maintained for 32 weeks. Also, the loss of jumping ability of the MPTP-treated marmosets in the Tower test was not recovered by 32 weeks. Positron emission tomography (PET) analysis revealed that remarkable decreases of bindings of 18F-FP-CIT were observed at the striatum of the brains of the marmosets received MPTP during the full period of the experiment for 32 weeks. In the substantia nigra of the marmosets, the loss of tyrosine hydroxylase (TH) immunoreactivity was also observed at 32 weeks following the MPTP treatment. In conclusion, our low-dose MPTP regimen protocol was found to be stable parkinsonism without mortality as evidenced by behavior, PET, and TH immunohistochemistry. This result will be useful for evaluation of possible long-term stem cell therapy for anti-parkinsonian activity.
Similar content being viewed by others
References
Khan W, Priyadarshini M, Zakai HA, Kamal MA, Alam Q. A brief overview of tyrosine hydroxylase and a-synuclein in the Parkinsonian brain. CNS Neurol Disord Drug Targets 2012;11:456–462.
Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol 2015;11:492–503.
Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep 2012;1:703–714.
Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011;480:547–551.
Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell 2015;16:269–274.
Offen D, Barhum Y, Levy YS, Burshtein A, Panet H, Cherlow T, et al. Intrastriatal transplantation of mouse bone marrow-derived stem cells improves motor behavior in a mouse model of Parkinson’s disease. J Neural Transm Suppl 2007;(72):133–143.
Sánchez-Pernaute R, Studer L, Bankiewicz KS, Major EO, McKay RD. In vitro generation and transplantation of precursor-derived human dopamine neurons. J Neurosci Res 2001;65:284–288.
Le W, Sayana P, Jankovic J. Animal models of Parkinson’s disease: a gateway to therapeutics? Neurotherapeutics 2014;11:92–110.
Blandini F, Armentero MT. Animal models of Parkinson’s disease. FEBS J 2012;279:1156–1166.
Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, et al. Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus. Cereb Cortex 2012;22:469–481.
Smith D, Trennery P, Farningham D, Klapwijk J. The selection of marmoset monkeys (Callithrix jacchus) in pharmaceutical toxicology. Lab Anim 2001;35:117–130.
Dell’Mour V, Range F, Huber L. Social learning and mother’s behavior in manipulative tasks in infant marmosets. Am J Primatol 2009;71:503–509.
Eslamboli A. Marmoset monkey models of Parkinson’s disease: which model, when and why? Brain Res Bull 2005;68:140–149.
Owen S, Thomas C, West P, Wolfensohn S, Wood M. Report on primate supply for biomedical scientific work in the UK. EUPREN UK Working Party. Lab Anim 1997;31:289–297.
Sasaki E. Prospects for genetically modified non-human primate models, including the common marmoset. Neurosci Res 2015;93:110–115.
Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, et al. Generation of transgenic non-human primates with germline transmission. Nature 2009;459:523–527.
Bezard E, Przedborski S. A tale on animal models of Parkinson’s disease. Mov Disord 2011;26:993–1002.
Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron 2010;66:646–661.
Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect Med 2011;1:a009316.
Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, et al. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res 1979;1:249–254.
Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A 1983;80:4546–4550.
Bezard E, Imbert C, Deloire X, Bioulac B, Gross CE. A chronic MPTP model reproducing the slow evolution of Parkinson’s disease: evolution of motor symptoms in the monkey. Brain Res 1997;766:107–112.
Duty S, Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol 2011;164:1357–1391.
Pearce RK, Jackson M, Britton DR, Shiosaki K, Jenner P, Marsden CD. Actions of the D1 agonists A-77636 and A-86929 on locomotion and dyskinesia in MPTP-treated L-dopa-primed common marmosets. Psychopharmacology (Berl) 1999;142:51–60.
Verhave PS, Vanwersch RA, van Helden HP, Smit AB, Philippens IH. Two new test methods to quantify motor deficits in a marmoset model for Parkinson’s disease. Behav Brain Res 2009;200:214–219.
Lundkvist C, Halldin C, Ginovart N, Swahn CG, Farde L. [18F] beta-CITFP is superior to [11C] beta-CIT-FP for quantitation of the dopamine transporter. Nucl Med Biol 1997;24:621–627.
Jenner P, Rupniak NM, Rose S, Kelly E, Kilpatrick G, Lees A, et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the common marmoset. Neurosci Lett 1984;50:85–90.
Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, et al. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 2001;76:1265–74.
Buchanan-Smith HM, Shand C, Morris K. Cage use and feeding height preferences of captive common marmosets (Callithrix j. jacchus) in twotier cages. J Appl Anim Welf Sci 2002;5:139–149.
Pearce RK, Jackson M, Smith L, Jenner P, Marsden CD. Chronic L-DOPA administration induces dyskinesias in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated common marmoset (Callithrix Jacchus). Mov Disord 1995;10:731–740.
Silverdale MA, Nicholson SL, Crossman AR, Brotchie JM. Topiramate reduces levodopa-induced dyskinesia in the MPTP-lesioned marmoset model of Parkinson’s disease. Mov Disord 2005;20:403–409.
Hantraye P, Loc’h C, Tacke U, Riche D, Stulzaft O, Doudet D, et al. “In vivo” visualization by positron emission tomography of the progressive striatal dopamine receptor damage occurring in MPTP-intoxicated nonhuman primates. Life Sci 1986;39:1375–1382.
Luquin MR, Manrique M, Guillén J, Arbizu J, Ordoñez C, Marcilla I. Enhanced GDNF expression in dopaminergic cells of monkeys grafted with carotid body cell aggregates. Brain Res 2011;1375:120–127.
Schwarz J. [Nuclear medicine imaging in patients with Parkinson’s syndrome: an update]. Nervenarzt 2010;81:1160–1167.
Nagai Y, Obayashi S, Ando K, Inaji M, Maeda J, Okauchi T, et al. Progressive changes of pre-and post-synaptic dopaminergic biomarkers in conscious MPTP-treated cynomolgus monkeys measured by positron emission tomography. Synapse 2007;61:809–819.
Saiki H, Hayashi T, Takahashi R, Takahashi J. Objective and quantitative evaluation of motor function in a monkey model of Parkinson’s disease. J Neurosci Methods 2010;190:198–204.
Masilamoni G, Votaw J, Howell L, Villalba RM, Goodman M, Voll RJ, et al. (18)F-FECNT: validation as PET dopamine transporter ligand in parkinsonism. Exp Neurol 2010;226:265–273.
Iravani MM, Haddon CO, Cooper JM, Jenner P, Schapira AH. Pramipexole protects against MPTP toxicity in non-human primates. J Neurochem 2006;96:1315–1321.
Iravani MM, Syed E, Jackson MJ, Johnston LC, Smith LA, Jenner P. A modified MPTP treatment regime produces reproducible partial nigrostriatal lesions in common marmosets. Eur J Neurosci 2005;21:841–854.
Jackson MJ, Jenner P. The MPTP-treated primate with specific reference to the use of the common marmoset (Callithrix jacchus). Neuromethods 2012;61:371–400.
Philippens IH, Wubben JA, Finsen B, Hart BA. Oral treatment with the NADPH oxidase antagonist apocynin mitigates clinical and pathological features of parkinsonism in the MPTP marmoset model. J Neuroimmune Pharmacol 2013;8:715–726.
Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, et al. UWA-121, a mixed dopamine and serotonin re-uptake inhibitor, enhances L-DOPA anti-parkinsonian action without worsening dyskinesia or psychosis-like behaviours in the MPTP-lesioned common marmoset. Neuropharmacology 2014;82:76–87.
Hurley MJ, Jackson MJ, Smith LA, Rose S, Jenner P. Proteomic analysis of striatum from MPTP-treated marmosets (Callithrix jacchus) with LDOPA-induced dyskinesia of differing severity. J Mol Neurosci 2014;52:302–312.
Hansard MJ, Jackson MJ, Smith LA, Rose S, Jenner P. A major metabolite of bupropion reverses motor deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated common marmosets. Behav Pharmacol 2011;22:269–274.
Johnston TH, Huot P, Fox SH, Wakefield JD, Sykes KA, Bartolini WP, et al. Fatty acid amide hydrolase (FAAH) inhibition reduces L-3,4-dihydroxyphenylalanine-induced hyperactivity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned non-human primate model of Parkinson’s disease. J Pharmacol Exp Ther 2011;336:423–430.
Jones CA, Johnston LC, Jackson MJ, Smith LA, van Scharrenburg G, Rose S, et al. An in vivo pharmacological evaluation of pardoprunox (SLV308)—a novel combined dopamine D(2)/D(3) receptor partial agonist and 5-HT(1A) receptor agonist with efficacy in experimental models of Parkinson’s disease. Eur Neuropsychopharmacol 2010;20:582–593.
Yabe H, Choudhury ME, Kubo M, Nishikawa N, Nagai M, Nomoto M. Zonisamide increases dopamine turnover in the striatum of mice and common marmosets treated with MPTP. J Pharmacol Sci 2009;110:64–68.
Nash JE, Ravenscroft P, McGuire S, Crossman AR, Menniti FS, Brotchie JM. The NR2B-selective NMDA receptor antagonist CP-101,606 exacerbates L-DOPA-induced dyskinesia and provides mild potentiation of anti-parkinsonian effects of L-DOPA in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp Neurol 2004;188:471–479.
Nomoto M, Kita S, Iwata SI, Kaseda S, Fukuda T. Effects of acute or prolonged administration of cabergoline on parkinsonism induced by MPTP in common marmosets. Pharmacol Biochem Behav 1998;59:717–721.
Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc 2007;2:141–151.
Potts LF, Wu H, Singh A, Marcilla I, Luquin MR, Papa SM. Modeling Parkinson’s disease in monkeys for translational studies, a critical analysis. Exp Neurol 2014;256:133–143.
Ando K, Obayashi S, Nagai Y, Oh-Nishi A, Minamimoto T, Higuchi M, et al. PET analysis of dopaminergic neurodegeneration in relation to immobility in the MPTP-treated common marmoset, a model for Parkinson’s disease. PLoS One 2012;7:e46371.
Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 2010;67:715–725.
Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest 2005;115:102–109.
Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, et al. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. J Parkinsons Dis 2011;1:395–412.
Yi BR, Kim SU, Choi KC. Development and application of neural stem cells for treating various human neurological diseases in animal models. Lab Anim Res 2013;29:131–137.
Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 2000;290:767–773.
Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience 2001;106:589–601.
Eidelberg E, Brooks BA, Morgan WW, Walden JG, Kokemoor RH. Variability and functional recovery in the N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of parkinsonism in monkeys. Neuroscience 1986;18:817–822.
Close SP, Elliott PJ, Hayes AG, Marriott AS. Effects of classical and novel agents in a MPTP-induced reversible model of Parkinson’s disease. Psychopharmacology (Berl) 1990;102:295–300.
Maneuf YP, Mitchell IJ, Crossman AR, Woodruff GN, Brotchie JM. Functional implications of kappa opioid receptor-mediated modulation of glutamate transmission in the output regions of the basal ganglia in rodent and primate models of Parkinson’s disease. Brain Res 1995;683:102–108.
Ando K, Maeda J, Inaji M, Okauchi T, Obayashi S, Higuchi M, et al. Neurobehavioral protection by single dose l-deprenyl against MPTP-induced parkinsonism in common marmosets. Psychopharmacology (Berl) 2008;195:509–516.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yun, JW., Ahn, JB., Kwon, E. et al. Behavior, PET and histology in novel regimen of MPTP marmoset model of Parkinson’s disease for long-term stem cell therapy. Tissue Eng Regen Med 13, 100–109 (2016). https://doi.org/10.1007/s13770-015-0106-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-015-0106-3