Abstract
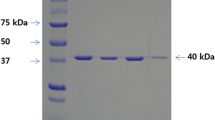
The Bacillus subtilis endo-β-1,4-glucanase gene (beg), which encodes the enzyme BEG that comprises 499 amino acid residues, was mutated by error-prone polymerase chain reaction and DNA shuffling to make variants with improved functionalities. The mutated DNAs were transformed into Escherichia coli DH5α, and among the 1370 transformants, a positive clone 8-20 was obtained finally based on a halo assay and thermostability and alkaline tolerance analyses. The mutated enzyme BEG8-20 of clone 8-20 was changed at seven amino acid residues compared to the wild-type enzyme BEGwt: K45E, I102Y, M112V, D226Y, D295E, L423S, and D460G. The optimum temperature and pH of BEG8-20 were nearly the same as those of BEGwt. However, the thermostability of BEG8-20 was increased 1.53-fold (21.4 min vs. 14.0 min) based on the half-life of the residual activity at 70 °C. This increase in enzyme thermostability is advantageous for the hydrolysis of lignocellulosic materials to produce fermentable sugars. In the activity staining experiment, only a truncated smaller enzyme was observed for BEG8-20, whereas two forms, a matured (52 kDa) and a truncated smaller (34.5 kDa) enzyme, were observed for BEGwt owing to proteolytic internal cleavage at the linker region between the 297th and 356th amino acid residues. These results indicate that amino acid substitutions in the mutant enzyme have rendered the protein prone to cleavage at the C-terminal region.




Similar content being viewed by others
References
Ahn DH, Kim H, Pack MY (1993) Cleavage of Bacillus subtilis endo-β-1,4-glucanase by B. megaterium protease. Biotechnol Lett 15:127–132
Akita M, Kayatama K, Hatada Y, Ito S, Horikoshi K (2005) A novel β-glucanase gene from Bacillus halodurans C-125. FEMS Microbiol Lett 248:9–15
Anbar M, Gul O, Lamed R, Sezerman UO, Bayer EA (2012) Improved thermostability of Clostridium thermocellum endoglucanase Cel8A by using consensus-guided mutagenesis. Appl Environ Microbiol 78:3458–3464
Cadwell RC, Joyce GF (1992) Randomization of genes by PCR mutagenesis. PCR Methods Appl 2:28–33
Cantwell BA, McConnell DJ (1983) Molecular cloning and expression of a Bacillus subtilis β-glucanase gene in Escherichia coli. Gene 23:211–219
Desphande MV, Eriksson KE, Pettersson LG (1984) An assay for selective determination of exo-1,4-beta-glucanases in a mixture of cellulolytic enzymes. Anal Biochem 138:481–487
Hanahan D (1985) Techniques for transformation of E. coli. In: Glover DM (ed) DNA cloning: a practical approach vol I. IRL Press, Oxford, pp 109–135
Kim H, Pack MY (1988) Endo-β-1,4-glucanase encoded by Bacillus subtilis gene cloned in Bacillus megaterium. Enzyme Microbiol Technol 10:347–351
Kim H, Kim SF, Ahn DH, Lee JH, Pack MY (1995) Internal cleavage of Bacillus subtilis BSE616 endo-β-1,4-glucanase expressed in Escherichia coli. J Microbiol Biotechnol 5:26–30
Kim IJ, Lee HJ, Choi IG, Kim KH (2014) Synergistic proteins for the enhanced enzymatic hydrolysis of cellulose by cellulase. Appl Microbiol Biotechnol 98:8469–8480
Kim DU, Kim HJ, Jeong YS, Na HB, Cha YL, Koo BC, Kim J, Yun HD, Lee JK, Kim H (2015) Enhanced saccharification of reed and rice straws by the addition of β-1,3-1,4-glucanase with broad substrate specificity and calcium ion. J Korean Soc Appl Biol Chem 58:29–33
Labrou NE (2010) Random mutagenesis methods for in vitro directed enzyme evolution. Curr Protein Pept Sci 11:91–100
Lee KD, Kim J, Kim H (1999) Purification and characterization of carboxymethyl-cellulase produced by Bacillus sp. KD1014. Agric Chem Biotechnol 42:107–112
Liang C, Fioroni M, Rodríguez-Ropero F, Xue Y, Schwaneberg U, Ma Y (2011) Directed evolution of a thermophilic endoglucanase (Cel5A) into highly active Cel5A variants with an expanded temperature profile. J Biotechnol 154:46–53
Lin L, Meng X, Liu P, Hong Y, Wu G, Huang X, Li C, Dong J, Xiao L, Liu Z (2009) Improved catalytic efficiency of endo-β-1,4-glucanase from Bacillus subtilis BME-15 by directed evolution. Appl Microbiol Biotechnol 82:671–679
Lin L, Fu C, Huang W (2016) Improving the activity of the endoglucanase, Cel8 M from Escherichia coli by error-prone PCR. Enzyme Microb Technol 86:52–58
Lo AC, MacKay RM, Seligy VL, Willick GE (1988) Bacillus subtilis β-1,4-endoglucanase products from intact and truncated genes are secreted into the extracellular medium by Escherichia coli. Appl Environ Microbiol 54:2287–2292
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
MacKay RM, Lo A, Willick G, Zuker M, Baird S, Dove M, Moranelli F, Seligy V (1986) Structure of a Bacillus subtilis endo-β-1,4-glucanase gene. Nucleic Acids Res 14:9159–9170
Mao S, Gao P, Lu Z, Lu F, Zhang C, Zhao H, Bie X (2016) Engineering of a thermostable β-1,3-1,4-glucanase from Bacillus altitudinis YC-9 to improve its catalytic efficiency. J Sci Food Agric 96:109–115
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination reducing sugar. Anal Chem 31:426–428
Ni J, Takehara M, Watanabe H (2005) Heterologous overexpression of a mutant termite cellulase gene in Escherichia coli by DNA shuffling of four orthologous parental cDNAs. Biosci Biotechnol Biochem 69:1711–1720
Park SH, Kim HK, Pack MY (1991) Characterization and structure of the cellulase gene of Bacillus subtilis BSE616. Agric Biol Chem 55:441–448
Robson LM, Chambliss GH (1987) Endo-β-1,4-glucanase gene of Bacillus subtilis DLG. J Bacteriol 169:2017–2025
Stemmer WP (1994) Rapid evolution of a protein in vitro by DNA shuffling. Nature 370:389–391
Telke AA, Zhuang N, Ghatge SS, Lee SH, Ali Shah A, Khan H, Um Y, Shin HD, Chung YR, Lee KH, Kim SW (2013) Engineering of family-5 glycoside hydrolase (Cel5A) from an uncultured bacterium for efficient hydrolysis of cellulosic substrates. PLoS ONE 8:e65727
Tiwari V (2016) In vitro engineering of novel bioactivity in the natural enzymes. Front Chem 4:39
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, MJ., Lee, H.W. & Kim, H. Enhancement of thermostability of Bacillus subtilis endoglucanase by error-prone PCR and DNA shuffling. Appl Biol Chem 60, 73–78 (2017). https://doi.org/10.1007/s13765-017-0254-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-017-0254-3