Abstract
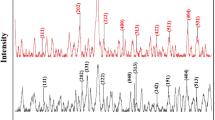
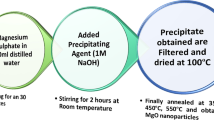
In this paper, graphene oxide–bismuth tungstate (GO–Bi2WO6) nanocomposite was synthesized and used as an efficient and green adsorbent for the removal of Pb2+. Bi2WO6 nanoplates were synthesized by the co-precipitation method and then used for modifying of graphene oxide. To characterize of the synthesized adsorbent, Fourier-transform infrared spectrophotometry (FT-IR), field emission scanning electron microscopy (FESEM), electron-dispersive X-ray spectroscopy (EDX) and X-ray diffraction spectroscopy (XRD) were used. By optimization of critical parameters including pH of sample solution, amounts of adsorbent and contact time, it was revealed that pH = 5.0, 20 mg adsorbent and 20 min contact time provide above 94% removal percentage for 50 mg L−1 Pb2+. Different adsorption isotherms including Langmuir, Freundlich, Temkin and Dubinin–Radushkevich were investigated, and the results show that the adsorption of Pb2+ followed by the Freundlich isotherm with a maximum adsorption capacity of 128 mg g−1. Also, interpretation of different kinetic models shows that adsorption of Pb2+ is followed by the pseudo-second-order kinetic model. Finally, the results of thermodynamic analysis show that adsorption of Pb2+ onto the GO–Bi2WO6 nanocomposite is endothermic and spontaneous process (ΔH ˃ 0, ΔG < 0) and desirable at higher temperatures.












Similar content being viewed by others
References
Al-Kinani A, Gheibi M, Eftekhari M (2019) Graphene oxide–tannic acid nanocomposite as an efficient adsorbent for the removal of malachite green from water samples. Model Earth Syst Environ 5:1627–1633
Arora R (2019) Adsorption of heavy metals: a review. Mater Today Proc 18:4745–4750
Bezzina JP, Robshaw T, Dawson R, Ogden MD (2020) Single metal isotherm study of the ion exchange removal of Cu(II), Fe(II), Pb(II) and Zn(II) from synthetic acetic acid leachate. Chem Eng J 394:124862
Boskabady M, Marefati N, Farkhondeh T, Shakeri F, Farshbaf A, Boskabady MH (2018) The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ Int 120:404–420
Brudey T, Largitte L, Jean-Marius C, Tant T, Couespel Dumesnil P, Lodewyckx P (2016) Adsorption of lead by chemically activated carbons from three lignocellulosic precursors. J Anal Appl Pyrolysis 120:450–463
Chandrashekhar Nayak M, Isloor AM, Inamuddin LB, Marwani HM, Khan I (2020) Polyphenylsulfone/multiwalled carbon nanotubes mixed ultrafiltration membranes: fabrication, characterization and removal of heavy metals Pb2+, Hg2+, and Cd2+ from aqueous solutions. Arab J Chem 13:4661–4672
Chen T, Liu L, Hu C, Huang H (2021) Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chin J Catal 42:1413–1438
Ciesielczyk F, Bartczak P, Jesionowski T (2016) Removal of cadmium(II) and lead(II) ions from model aqueous solutions using sol–gel-derived inorganic oxide adsorbent. Adsorption 22:445–458
Eftekhari M, Akrami M, Gheibi M, Azizi-Toupkanloo H, Fathollahi-Fard AM, Tian G (2020) Cadmium and copper heavy metal treatment from water resources by high-performance folic acid-graphene oxide nanocomposite adsorbent and evaluation of adsorptive mechanism using computational intelligence, isotherm, kinetic, and thermodynamic analyses. Environ Sci Pollut Res 27:43999–44021
Eftekhari M, Gheibi M, Azizi-Toupkanloo H, Hossein-Abadi Z, Khraisheh M, Fathollahi-Fard AM, Tian G (2021) Statistical optimization, soft computing prediction, mechanistic and empirical evaluation for fundamental appraisal of copper, lead and malachite green adsorption. J Ind Inf Integr 23:100219
Eftekhari M, Gheibi M, Monhemi H, Gaskin Tabrizi M, Akhondi M (2022) Graphene oxide-sulfated lanthanum oxy-carbonate nanocomposite as an adsorbent for the removal of malachite green from water samples with application of statistical optimization and machine learning computations. Adv Powder Technol 33:103577
El-Naggar IM, Ahmed SA, Shehata N, Sheneshen ES, Fathy M, Shehata A (2019) A novel approach for the removal of lead (II) ion from wastewater using Kaolinite/Smectite natural composite adsorbent. Appl Water Sci 9:7–19
Fu R, Liu Y, Lou Z, Wang Z, Baig SA, Xu X (2016) Adsorptive removal of Pb(II) by magnetic activated carbon incorporated with amino groups from aqueous solutions. J Taiwan Inst Chem Eng 62:247–258
Ghadirimoghaddam D, Gheibi M, Eftekhari M (2021) Graphene oxide-cyanuric acid nanocomposite as a novel adsorbent for highly efficient solid phase extraction of Pb2+ followed by electrothermal atomic absorption spectrometry; statistical, soft computing and mechanistic efforts. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1861260
Harikumar PS, Hridya TK (2021) Application of CuNi bimetallic nanoparticle as an adsorbent for the removal of heavy metals from aqueous solution. Inter J Environ Anal Chem 101:869–883
Hernández-Francisco E, Bonilla-Cruz J, Márquez-Lamas U, Suárez-Jacobo A, Longoria-Rodríguez F, Rivera-Haro J, Russell P, Ali Z, Yin CY, Lara-Ceniceros TE (2020) Entangled cellulose nanofibrils/nanosheets derived from native mexican agave for lead(II) ion removal. Cellulose 27:8785–8798
Ho SH, Yang Z, Nagarajan D, Chang JS, Ren N (2017) High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour Technol 246:142–149
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72
Kataria N, Garg VK (2018) Optimization of Pb (II) and Cd (II) adsorption onto ZnO nanoflowers using central composite design: isotherms and kinetics modelling. J Mol Liq 271:228–239
Kinuthia GK, Ngure V, Beti D, Lugalia R, Wangila A, Kamau L (2020) Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: community health implication. Sci Rep 10:8434–8446
Laabd M, Ait Ahsaine H, El Jaouhari A, Bakiz B, Bazzaoui M, Ezahri M, Albourine A, Benlhachemi A (2016) Congo red removal by PANi/Bi2WO6 nanocomposites: kinetic, equilibrium and thermodynamic studies. J Environ Chem Eng 4:3096–3105
Latif Wani A, Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8:55–64
Leudjo Taka A, Fosso-Kankeu E, Pillay K, Yangkou Mbianda X (2018) Removal of cobalt and lead ions from wastewater samples using an insoluble nanosponge biopolymer composite: adsorption isotherm, kinetic, thermodynamic, and regeneration studies. Environ Sci Pollut Res 25:21752–21767
Nemati M, Hosseini SM, Shabanian M (2017) Novel electrodialysis cation exchange membrane prepared by 2-acrylamido-2-methylpropane sulfonic acid; heavy metal ions removal. J Hazard Mater 337:90–104
Rezazadeh N, Danesh S, Eftekhari M (2021a) Efficient removal of Triton X-100 from water samples by graphene oxide-humic acid nanocomposite. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1900145
Rezazadeh N, Danesh S, Eftekhari M (2021b) TX-100 adsorption from aqueous solution using modified graphene oxide; optimization by response surface methodology and one factor at a time techniques. J Dispers Sci Technol. https://doi.org/10.1080/01932691.2021.1979409
Rezazadeh N, Eftekhari M, Akhondi M, Aljalawee EAJ (2022) Novel graphene oxide-polyethylene glycol mono-4-nonylphenyl ether adsorbent for solid phase extraction of Pb2+ in blood and water samples. J Environ Health Sci Eng. https://doi.org/10.1007/s40201-022-00807-0
Safa Ozcan A, Gok O, Ozcan A (2009) Adsorption of lead(II) ions onto 8-hydroxy quinoline-immobilized bentonite. J Hazard Mater 161:499–509
Sall ML, Diaw AKD, Gningue-Sall D, Efremova Aaron S, Aaron JJ (2020) Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ Sci Pollut Res 27:29927–29942
Sani HA, Ahmad MB, Hussein MZ, Ibrahim NA, Musa A, Saleh TA (2017) Nanocomposite of ZnO with montmorillonite for removal of lead and copper ions from aqueous solutions. Process Saf Environ Prot 109:97–105
Sheshmani S, Nejabat Ghamsari H (2020) Photodegradation of acid orange 7 from aqueous solution under visible light irradiation using nanosized ZnO/chitosan/graphene oxide composite. Inter J Environ Anal Chem 100:912–921
Wang D, Zhang J, Guo L, Dong X, Shen H, Fu F (2016) Synthesis of nano-porous Bi2WO6 hierarchical microcrystal with selective adsorption for cationic dyes. Mater Res Bull 83:387–395
Wu S, Sun J, Li Q, Hood ZD, Yang S, Su T, Peng R, Wu Z, Sun W, Kent PRC, Jiang B, Chisholm MF (2020) Effects of surface terminations of 2D Bi2WO6 on photocatalytic hydrogen evolution from water splitting. ACS Appl Mater Interfaces 12:20067–20074
Xu S, Jiang X, Liu L, Wang Z, Zhang X, Peng Y, Cao M (2019) Preparation of PVA/tetra-ZnO composite with framework-supported pore-channel structure and the removal research of lead ions. Environ Sci Pollut Res 26:24062–24074
Zhang Z, Wang W, Shang M, Yin W (2010) Low-temperature combustion synthesis of Bi2WO6 nanoparticles as a visible-light-driven photocatalyst. J Hazard Mater 177:1013–1018
Zhao G, Liu S, Lu Q, Song L (2012) Controllable synthesis of Bi2WO6 nanofibrous mat by electrospinning and enhanced visible photocatalytic degradation performances. Ind Eng Chem Res 51:10307–10312
Zhi-Hang W, Bao-Yu Y, Jie T, Fei-Peng J, Xin-Yu J, Jin-Gang Y, Ming Z, Xiao-Qing C (2016) Tartaric acid modified graphene oxide as a novel adsorbent for high-efficiently removal of Cu(II) and Pb(II) from aqueous solutions. J Taiwan Inst Chem Eng 66:181–190
Zhu D, Zhou Q (2020) Novel Bi2WO6 modified by N-doped graphitic carbon nitride photocatalyst for efficient photocatalytic degradation of phenol under visible lightAppl. Catal B 268:118426
Acknowledgments
Authors wish to thank the University of Neyshabur for its financial support (Grant Number: 25783).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declared that they do not have any conflict of interest.
Additional information
Editorial responsibility: R Saravanan.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saadati, T., Eftekhari, M., Rezazadeh, N. et al. Graphene oxide–bismuth tungstate (GO–Bi2WO6) nanocomposite as a green adsorbent for lead removal: isotherm, kinetics and thermodynamic study. Int. J. Environ. Sci. Technol. 20, 1301–1314 (2023). https://doi.org/10.1007/s13762-022-04627-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04627-5