Abstract
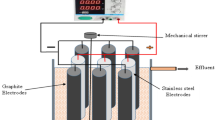
Laboratory experiments were carried out to investigate the mechanisms of electrochemical disinfection of artificial wastewater contaminated by Escherichia coli culture (5 × 105 UFC/100 mL) using electrocoagulation. In order to go deeply into the mechanism of the process, the behaviors of two dissolved-type electrodes (ordinary steel and aluminum) and a non-dissolved-type (carbon graphite) electrode were compared. The ordinary steel electrode was found more efficient for E. coli cells destruction compared to aluminum and carbon graphite electrodes. In order to determine the most favorable condition for the treatment, the effect of various supporting electrolytes including, sodium chloride, sodium sulfate and sodium nitrate, was scrutinized. E. coli is inactivated by 5 log units for a charge loading of 37.30 F/m3 for sodium sulfate, 24.87 F/m3 for sodium nitrate and 12.43 F/m3 for sodium chloride. It thus appears that the most favorable supporting electrolyte type for this method of disinfection is sodium chloride, a fact which can be explained by the formation of disinfectant by-products such as chlorine dioxide, hypochlorite ions and perchlorate ions. From the results obtained, electrocoagulation applied to the elimination of E. coli proceeds through three combined effects: the electric field, the actions of oxidants electrogenerated during the process and the adsorption by the metallic hydroxides formed in solution.






Similar content being viewed by others
References
Abu-Shkara F, Neeman I, Sheinman R, Armon R (1998) The effect of fatty acid alteration in coliform bacteria on disinfection resistance and/or adaptation. Water Sci Technol 38(12):133–139
Bergmann MEH, Koparal AS (2005) Studies on electrochemical disinfectant production using anodes containing RuO2. J Appl Electrochem 35(12):1321–1329
Bergmann MEH, Rollin J (2007) Product and by-product formation in laboratory studies on disinfection electrolysis of water using boron-doped diamond anodes. Catal Today 124(3–4):198–203
Birbir M, Hacioğlu H, Birbir Y, Altuğ G (2009) Inactivation of Escherichia coli by alternative electric current in rivers discharged into sea. J Electrostat 67(4):640–645
Bull NB, Krasner SW, Daniel P, Bull RD (2001) Health effects and occurrence of disinfection by-products. AWWA Research foundation and American Water Works Association
Cañizares P, Larrondo F, Lobato J, Rodrigo MA, Sáez C (2005) Electrochemical synthesis of peroxodiphosphate using boron-doped diamond anodes. J Electrochem Soc 152(11):191–196
Chen X, Chen G, Yue PL (2000) Separation of pollutants from restaurant wastewater by electrocoagulation. Sep Purif Technol 19(1–2):65–76
Diao HF, Li XY, Gu JD, Shi HC, Xie ZM (2004) Electron microscopic investigation of the bactericidal action of electrochemical disinfection in comparison with chlorination, ozonation and Fenton reaction. Process Biochem 39(11):1421–1426
Drees KP, Abbaszadegan M, Maier RM (2003) Comparative electrochemical inactivation of bacteria and bacteriophage. Water Res 37(10):2291–2300
Driedger AM, Rennecker JL, Marinas BJ (2000) Sequential inactivation of Cryptosporidium parvum oocysts with ozone and free chlorine. Water Res 34(14):3479–3493
Entwisle JH (1974) Consumption of graphite anodes in chlorine manufacture by brine electrolysis. J Appl Electrochem 4(4):293–303
Essadki AH, Bennajah M, Gourich B, Vial Ch, Azzi M, Delmas H (2008) Electrocoagulation/electroflotation in an external-loop airlift reactor-application to the decolorization of textile dye wastewater: a case study. Chem Eng Proc Process Intensif 47(8):1211–1223
Furuta T, Tanaka H, Nishiki Y, Pupunat L, Haenni W, Rychen P (2004) Legionella inactivation with diamond electrodes. Diamond Relat Mater 13:2016–2019
Gao S, Du M, Tian J, Yang J, Yang J, Ma F, Nan J (2010) Effects of chloride ions on electro-coagulation–flotation process with aluminium electrodes for algae removal. J Hazard Mater 182(1–3):827–834
Ghernaout D, Badis A, Kellil A, Ghernaout B (2008) Application of electrocoagulation in Escherichia coli culture and two surface waters. Desalination 219(1–3):118–125
Igne V (1998) ‘Traitement des effluents liquides par electrocoagulation-flocculation. Dossier d’actualisation (CFE), centre français d’électricité-pôle technique et veille, Paris, 8–19
Izquierdo CJ, Cañizares P, Rodrigo MA, Leclerc JP, Valentin G, Lapicque F (2010) Effect of the nature of the supporting electrolyte on the treatment of soluble oils by electrocoagulation. Desalination 255(1–3):15–20
Jeong J, Kim JY, Yoon J (2006) The role of reactive oxygen species in the electrochemical inactivation of microorganisms. Environ Sci Technol 40(19):6117–6122
Jeong J, Kim C, Yoon J (2009) The effect of electrode material on generation of oxidants and microbial inactivation in the electrochemical disinfection processes. Water Res 43(4):895–901
Kerwick MI, Reddy SM, Chamberlin AHL, Holt DM (2005) Electrochemical disinfection, an environmentally acceptable method of drinking water disinfection? Electrochim Acta 50(25–26):5270–5277
Kumar PR, Chaudhari S, Khilar KC, Mahajan SP (2004) Removal of arsenic from water by electrocoagulation. Chemosphere 55(9):1245–1252
Li M, Qu JH, Peng YZ (2004) Sterilization of E. coli cells by the application of pulsed magnetic field. J Environ Sci 16(2):348–352
Longley KE (1986) Wastewater disinfection, manuals of practice for water pollution control. Water pollution control federation, Alexandria
Martinez-Huitle CA, Brillas E (2008) Electrochemical alternatives for drinking water disinfection. Angwante Chemie 47(11):1998–2005
Mollah MYA, Morkovskyb P, Gomes JAG, Kesmez M, Pargad J, Cocke DL (2004) Fundamentals, present and future perspectives of electrocoagulation. J Hazard Mater 114(1–3):199–210
Nanseu-Njiki CP, Tchamango SR, Ngom PC, Darchen A, Ngameni E (2009) Mercury(II) removal from water by electrocoagulation using aluminium and iron electrodes. J Hazard Mater 168(2–3):1430–1436
Panizza M (2010) Importance of electrode material in the electrochemical treatment of wastewater containing organic pollutants (ch. 2). In: Comninellis C, Chen G (eds) Electrochemistry for the environment. Springer Science + Business Media, LLC, New York, pp 25–54
Pouet MF, Grasmick A (1995) Urban wastewater treatment by electrocoagulation and flotation. Water Sci Technol 31(3–4):275–287
Sharma AK, Venkobachar C (1979) Effect of prechlorination on coagulation of algae. India J Environ Health 21(1):16–22
Sloat S, Ziel C (1992) The use of indicator organisms to assess public water safety. Technical Information series-Booklet No.13. HACH technical centre for applied analytical chemistry, HACH Compagny, Loveland, Colorado USA, 1–15
Trompette JL, Vergnes H (2009) On the crucial influence of some supporting electrolytes during electrocoagulation in the presence of aluminum electrodes. J Hazard Mater 163(1–2):1282–1288
Vega-Mercado H, Pothakamury UR, Chang FJ, Barbosa-Cánovas GV, Swanson BG (1997) Inactivation of E. coli by combining pH, ionic strength and pulsed electric fields hurdles. Food Res Int 29(2):117–121
Yildiz YS, Koparal AS, Keskinler B (2008) Effect of initial pH and supporting electrolyte on the treatment of water containing high concentration of humic substances by electrocoagulation. Chem Eng J 138(1–3):63–72
Zhu B, Clifford DA, Chellam S (2005) Comparison of electrocoagulation and chemical coagulation pretreatment for enhanced virus removal using microfiltration membranes. Water Res 39(13):3098–3108
Acknowledgments
The authors wish to thank the International Foundation of Science (IFS) and the Organization for Prohibition of Chemical Weapons (OPCW) (Project No. W/4395-1, Dr Nanseu-Njiki C. P.) for their financial support. They also thank AIRES-Sud, a program from the French Ministry of Foreign and European Affairs implemented by the Institut de Recherche pour le Developpement (IRD-DSF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ndjomgoue-Yossa, A.C., Nanseu-Njiki, C.P., Kengne, I.M. et al. Effect of electrode material and supporting electrolyte on the treatment of water containing Escherichia coli by electrocoagulation. Int. J. Environ. Sci. Technol. 12, 2103–2110 (2015). https://doi.org/10.1007/s13762-014-0609-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0609-9