Abstract
Background
Falls represent a critical concern in Parkinson’s disease (PD), contributing to increased morbidity and reduced quality of life.
Purpose
We conducted a systematic review to assess the prognostic factors associated with falls in PD, aiming to provide a comprehensive overview of relevant demographic and clinical parameters, and aid neurologists in identifying subsets of PD patients most susceptible to falls and associated injuries.
Methods
PubMed and Web of Science databases were searched for prospective studies assessing factors associated with falls in ambulatory PD patients across different settings, from inception to August 2023. Data extraction was conducted using CHARMS-PF checklist and risk of bias was assessed with QUIPS tool. PRISMA guidelines were followed.
Results
The initial search yielded 155 references. Thirty-four studies, involving a total of 3454 PD patients, were included in the final analysis. The mean pooled age was 67.6 years, and 45.1% were women. PD patients presented mild motor impairment (UPDRS III score 27.8) with mean pooled disease duration of 5.7 years. Gait and balance disorders and history of prior falls emerged as the most consistent predictors of falls across studies. Disease duration, disease severity, dysautonomic symptoms, freezing of gait, frontal cognitive functions, and PD medication dosages yielded inconsistent findings. Conversely, dyskinesias, age, sex, and depression were unrelated to future falls in PD. Logistic regression models were most commonly employed to identify factors significantly associated with falls in PD. Substantial heterogeneity prevailed in the inclusion of confounding factors.
Conclusion
The evidence suggests that previous history of falls, gait disorders, and poor balance are robust prognostic markers for falls in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Falls are involuntary incidents that disrupt balance and result in the body coming into contact with the ground or another solid surface. They pose a significant health problem, particularly in individuals aged 65 and above [1], with an even higher prevalence among those suffering from Parkinson’s disease (PD) [2]. As the population continues to age, falls become an increasingly pressing challenge for public health worldwide [3]. It is estimated that between 10 and 35% of falls in this age group lead to fractures, often requiring hospitalization, with hip fractures being the most common at 10% incidence rate [4, 5].
In patients with PD, the annual incidence rate of falls ranges from 45 to 68%, which is three times higher than in healthy individuals [6, 7]. Approximately 50% of falls result in severe secondary injuries, underscoring the importance of identifying the underlying factors contributing to falls to minimize their occurrence [8]. Developing and optimizing therapeutic strategies to prevent falls requires identifying PD patients at risk for falling. Factors that have been identified as potential risk indicators for falls include previous history of falls, occurrence of freezing of gait (FoG), cognitive decline, compromised postural stability and balance, diminished lower limb strength, and reduced gait velocity [7, 9,10,11,12,13]. However, it is important to note that some of these studies are retrospective in nature, potentially susceptible to recall bias. Therefore, a comprehensive review of the current body of evidence derived from prospective studies is warranted to establish robust prognostic indicators for falls in PD. Thus, the aim of this systematic review was to determine the most significant prognostic factors for falls in ambulatory PD patients identified in prospective studies.
Methods
Protocol
We designed the systematic review according to the PICOTS system, and reported the results in accordance with Preferred Reporting Items for Systematic reviews and Meta-Analysis Protocols (PRISMA-P) 2015 Statement [14]. The protocol was registered with PROSPERO (registration number CRD42023437145).
Ethical considerations
Our study only included anonymized data and no personal information was handled or any procedure applied to human beings, therefore, the ethical approval was not required.
Data sources and search strategy
The search for systematic review was performed in MEDLINE database (via Pubmed) and ISI Web of Knowledge (see Supplemental File for search strategy). The search included articles from database inception to 15th August 2023, with additional articles identified from reference checking [15]. No language restrictions were applied.
Two independent researchers (A.M. and O.M.) screened all titles and abstracts resulting from the electronic databases using Rayyan software [16]. Whole manuscripts were reviewed for article selection based on eligibility criteria. Discrepancies in article selection were addressed via discussion.
Study selection and inclusion criteria
Articles were eligible for inclusion if their primary aim was to assess the potential association between a candidate prognostic factor (including demographics, psychometric, biometric or others) and risk of future falls in patients with PD recruited in any setting. Further inclusion criteria were as follows: (1) original articles; (2) longitudinal cohort or case–control studies without limitations of follow-up time; (3) prospective studies. Studies including PD patients with Deep Brain Stimulation, patients who required the use of a wheelchair or whose Hoehn & Yahr (H&Y) stage was 5 were excluded.
Data extraction
Data from included studies were extracted in a spreadsheet based on CHARMS-PF checklist [17]. We gathered information on the following aspects: (1) Source of data; (2) Sample characteristics (eligibility criteria, demographics and clinical variables); (3) Outcome definition, measurement, and timing; (4) Number, and type of measurement of predictors; (5) Sample size; (6) Missing data in outcome or predictors variables and handling of missing data; (7) Summary of results including the non-adjusted and adjusted estimates of statistical analyses, statistical significance, and the included confounding factors.
Quality assessment
We used The Quality in Prognosis Studies (QUIPS) tool [18] to appraise the quality of included prognostic studies. The QUIPS tool consists of six domains that assess potential sources of bias conducting prognostic studies: patient selection, study attrition, measurement of prognostic factors, outcome measurement, confounding factors, and statistical analysis and results presentation. The adapted QUIPS tool for the current systematic review is provided in Supplemental Material.
Data synthesis
We assumed heterogeneity on study design, methodological quality, methods for prognostic factor assessment, duration of follow-up, statistical analyses and data presentation across studies. Therefore, a qualitative synthesis of the available evidence was performed. To assess the certainty in the body of evidence of an outcome, the frequency of finding a significant association was assessed, taking into account both sample size and patient characteristics.
Results
Study selection and characteristics
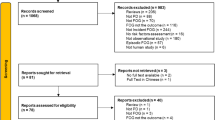
Figure 1 depicts the study selection procedure. The search query retrieved 62 references in PubMed and 61 references in WoS. None of the retrieved papers were in a non-English language. After duplicate removal and screening step, the full text of one article could not be retrieved [19], and thus, 35 references were selected for full text review. Additionally, 32 references were identified by screening reference lists of the selected publications. Table 1 shows the characteristics of the 34 included studies. From these, 31 were cohort studies and 3 were case–control longitudinal studies [20,21,22]. The follow-up time varied including follow-ups at 3 months [23], 6 months [21, 22, 24,25,26,27,28,29], 1 year [10, 20, 28, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45], 2 years [46], 2 years and a half [47,48,49], 3 years and a half [50, 51], and 1 study with follow-ups up to 8 years [52].
Patient characteristics
The total number of included PD patients with follow-ups was 3454. Demographic and clinical characteristics of patients are reported in Table 2. The smallest sample size consisted of 26 patients [46] and the largest one of 305 PD patients [28] who pooled the data from two cohorts [10, 25]. The pooled mean age was 67.6 years old and 45.1% were female PD patients. Disease duration ranged from 0 to 10.2 years (pooled mean 5.7 years), although 8 out of 34 (22.8%) studies did not report this value [10, 20, 21, 23, 28, 33, 37, 45]. Regarding disease stage, Hoenh & Yahr (H&Y) was available in 25 (71.4%) studies, the median ranging from 2 to 3. The pooled mean Unified Parkinson’s Disease Rating Scale (UPDRS), part III score (available in 85.3% of studies) was 27.8. Global cognitive scores were available in 21 out of 34 studies (61.7%), most of them using Mini–Mental State Examination (MMSE), although 2 studies assessed cognition with Montreal Cognitive Assessment (MoCA) [22, 42]. The average cognitive score was normal in all studies for PD patients.
Outcome definition and measurement
Falls were defined in most studies as “an unexpected event in which the person comes to rest on the ground, floor, or lower level” or a similar definition. However, seven studies did not provide a specific definition for falls [20, 22, 33, 35, 39, 43, 49]. In all studies, for quantifying or assessing falls in PD patients, the participants prospectively recorded falls on a diary or calendar. The researchers contacted the participants during follow-up by phone or by face to face interviews to register fall incidence. Five studies used recurrent falls as the unique outcome, two studied falls and recurrent falls, one the occurrence of first fall, and two studied falls and near falls (Table 1).
Statistical models
Most studies used logistic regression to determine the factors associated with falling. Some of these studies extended their analyses using ROC Curves to assess model performance. Two studies opted for multivariate Poisson regression [22, 51], while one study employed negative binomial regression [30] to identify factors associated with the frequency of falls. In contrast, another study used Cox Proportional Hazard analysis [48] to assess the time from study enrollment to first fall. Furthermore, a chi-squared test [23] was used for exploratory analyses of factors that increased the risk of falls.
Confounding factors
Twenty-six studies took confounding factors into consideration. The most frequently considered confounding factors encompassed age, sex, parameters related to walking (such as stride length or gait cycle time), prior fall history, disease duration, disease severity (measured by means of H&Y or UPDRS III scale), FoG, depression and anxiety levels, physical activity, levodopa-equivalent daily dose (LEDD, mg/day), or balance assessments (Activities-specific Balance Confidence [ABC] or Tinetti scales). The confounding factors considered in each study varied both in terms of quantity and the specific factors that were included, depending on the adopted criteria in each work. For instance, certain studies used a criterion whereby a confounding factor needed to achieve a specific level of statistical significance in univariate analyses before being incorporated into multivariable analyses.
Factors associated with falls
The most extensively investigated prognostic factors for falls were history of falls, balance and gait, and FoG, followed by gait parameters, disease duration, disease severity, and global cognition (Fig. 2). Demographic factors were considered in less than 50% of included studies.
History of falls
In relation to the history of falls, 14 studies examined this predictive variable, with the majority of them reaching the consensus that prior falls were linked to subsequent fall incidents [10, 20, 22, 25, 35, 40, 44, 50, 51, 53]. Nevertheless, it is noteworthy that the fear of falling did not exhibit an association with falls [28, 40, 41, 46, 49].
Balance and gait parameters
The most extensively employed clinical evaluations for gait and balance include the 10-m walking test (10-MWT) [21, 36, 50, 51], which evaluates mobility at comfortable and maximal walking speed, Timed Up and Go test [10, 21, 31, 42], the Tinetti Balance Assessment evaluating both gait and balance [21, 46, 49], the 7-item Berg Balance Scale quantifying static and dynamic balance during specific movement tasks [21, 27, 31], Functional Reach Test measuring the maximal distance an individual can reach forward from a standing position [21, 25, 31], Activities-specific Balance Confidence (ABC) scale [31, 34, 40, 41, 43, 48], Mini-Balance Evaluation System Test (Mini-BESTest) [27, 29, 37, 41], Dynamic Gait Index assessing adaptability of balance during ambulation in the presence of external demands [31], and the Retropulsion test [50, 51]. Gait and balance were assessed in 21 studies.
In the included articles, regardless of the chosen assessment tool or questionnaire, a consensus emerged from 11 out of 16 studies assessing balance [21, 27, 29, 33, 34, 37, 41, 43, 46, 48, 51], and from 9 out of 12 studies assessing gait [10, 21, 22, 24, 46, 48, 50, 51, 53]. These studies showed that gait disorder and poor balance were associated with future falls. On the other hand, balance confidence measured with ABC-16 questionnaire consistently demonstrated to be significantly associated with falls [34, 40, 41, 43]. The one study that failed to find such an association was the one whose outcome was the occurrence of first fall [48].
In our systematic review, a variety of gait measurement methods have been employed, with particular focus on the instrumental evaluations conducted by Lord [48] and Hoskovcová [22]. These evaluations comprehensively assessed gait parameters and revealed a strong association between gait speed and the likelihood of future falls. Conversely, Duncan et al. [53] assessed gait using the 10-MWT, while Paul et al. [25] based their evaluation on self-selected walking pace. Both studies concluded that these gait parameters serve as reliable indicators for predicting falls. However, a study conducted by Kataoka et al.. [46] suggested that gait speed alone may not be a reliable predictor of falls. It is important to highlight that this particular study had a small sample size of only 26 participants, which could potentially constrain the ability to formulate definitive conclusions.
Freezing of Gait
Thirteen studies explored the association between FoG and future falls. The FoGQ scale emerged as the predominant assessment tool within the reviewed articles for analyzing FoG [29, 41, 50, 53], although certain studies alternatively employed UPDRS II item 14 [22, 31, 52] or inquired about prior instances of FoG [10, 20]. However, the findings concerning its predictive efficacy for falls were contradictory. A study using Falls Efficacy Scale International Questionnaire (FES-IQ) [35], history of FoG [10, 20], FoG item from UPDRS scale [31, 52] and one study using FoG Questionnaire [25], proved FoG to be significant predictors of falls. Contrarily, half of the studies yielded opposing outcomes. As a result, the association between FoG and falls remains unclear and additional research in this area is required.
Demographic factors
Demographic factors have not been included in many studies, but those introducing them as confounding variables have concluded that age and sex were not significantly related to falls [10, 22, 26, 30, 39, 41]. Other demographic factors, such as the socioeconomic status, have not been explored in the selected articles.
Disease-related variables
In addition to biometric measurements, several studies have investigated the predictive nature of disease-related variables in relation to falls. These variables include dyskinesia, disease duration, motor severity, disease stage, postural asymmetry, and medication. Dyskinesias disrupt motor control and gait patterns and, therefore, have been considered as prognostic factor for falls. However, in this systematic review, the four studies including dyskinesias as prognostic factor concluded that they were not predictors of falls [22, 35, 50, 51]. However, there is conflicting information regarding the predictive role of disease duration or severity. All incorporated studies concur that the H&Y scale serves as a valuable tool for predicting falls [20, 30, 48], whereas the ability of UPDRS III in isolation to prognosticate falls is still controversial [30, 40, 41, 49]. In regard to disease duration, divergent outcomes emerge, and it is difficult to draw conclusion from the current evidence [22, 35, 39, 44, 47]. One study focusing on postural asymmetry concluded that it was a predictor of falls [33]. Out of the five studies including LEDD as a prognostic factor for falls, three studies concluded that future fallers tend to have higher initial doses of levodopa [22, 39, 52], and one study concluded that the proportion of fallers with LEDD < 500 at baseline was significantly smaller [10], whereas in the remaining study [31] the daily LEDD was near significance for predicting recurrent fallers.
Non-motor symptoms
This review also gathered information about neuropsychological and other non-motor symptoms as prognostic factors for falls. The most prominent symptoms analyzed were cognition, depression, and dysautonomic symptoms. Executive functions were found to be predictive of falls in five out of eight studies that assessed it [28, 30, 41, 46, 49], whereas global cognition found not to be a significant prognostic factor for falls in seven out of eleven studies [20, 22, 35, 46, 48, 49, 52]. PD patients with cardiac autonomic neuropathy [42] or mild urinary urgency [26] seem to be at higher risk of falls. Contrarily, dizziness or the presence of symptoms of orthostatic hypotension were not predictors of falls [10, 23, 30, 39, 44, 48]. Regarding depression, five studies analyzing this factor as a prognostic indicator for falls found that depression was not related to falls [28, 40, 41, 48], except one study showing the opposite result [39]. Lastly, Gazibara et al.. [38, 45] reported that health-related quality of life questionnaire could also predict the occurrence of falls within 1 year of follow-up. Specifically, PD patients reporting poor physical functioning or vitality in the questionnaire were at higher risk of falls.
Quality of included studies
Using the QUIPS tool, 18 studies scored high at least in one domain from which 3 studies scored high in two domains [20, 39, 48]. The most frequently noted sources of high bias were Study Attrition (n = 8), and Confounding Factors (n = 6) categories. Confounding bias occurred when studies did not adjust for relevant, potentially confounding variables in multivariable models. Studies without an adequate strategy to address substantial missing data accounted for Study Attrition bias. Moderate risk of bias was observed in 17 studies regarding Study Participant category. Sources of bias related to study participation were generally due to insufficient clinical description of the sample (Fig. 3).
Discussion
In this systematic review, we reviewed the current literature, specifically focusing on prospective studies that met our inclusion criteria, to identify the factors predicting falls in PD. From the interpretation of the included studies we have reached several conclusions: (1) strong evidence was found for poor balance, gait disorders, and history of falls at baseline as prognostic factors for falls in PD; (2) our review showed limited and conflicting evidence with regard to FoG, disease severity, disease duration or frontal cognitive abilities as potential predictors for falls; (3) no evidence was found for the association of baseline dyskinesias, global cognitive impairment, orthostatic symptoms, age or sex with future falls.
Previous systematic reviews have explored fall-related factors but span more than a decade and were limited by the number of articles included. Notably, Pickering et al. in 2007 [54] examined six prospective studies, and Allen et al. [55] updated the literature in 2013 with 15 prospective and 11 retrospective studies. Both systematic reviews concluded that the history of falls was the main predictor for falls in PD. They also explored the influence of disease severity, ultimately concluding that its contribution to fall prediction was minimal, which is in line with our results. However, these early reviews lacked an exploration of the full spectrum of potential predictors, which underscores the importance of revisiting this topic with a broader scope. In the current systematic review, we expand on these prior reviews by incorporating a more substantial number of prospective studies conducted in the last decade. In this work, in contrast to previous ones, we explored the role of demographics and other non-motor symptoms, including cognition, autonomic nervous system dysfunction or depression.
In the selected 34 articles, more than 17 prognostic factors were analyzed, with notable emphasis on the history of falls, FoG, balance, and gait disorders. However, not all the included articles specified the definition of falls. Notably, seven of them lack a clear definition of falls [20, 22, 33, 35, 39, 43, 49], which may limit the interpretability of the results. Moreover, some studies used “repetitive falls”, “near-falls,” or “the occurrence of first fall” as the primary outcome. Therefore, the prognostic factors identified in each study might not necessarily coincide, given the subtle variations in outcome definition. Additionally, some studies did not account for confounding factors, while those that did incorporate them displayed substantial heterogeneity in the quantity and nature of variables considered in multivariable analyses, rendering the comparability between studies challenging.
Gait and balance assessment have emerged as pivotal functional parameters frequently analyzed in PD for their predictive capacity concerning falls. In 2018, Creaby et al. [56] investigated the biomechanical parameters associated with falls in a meta-analysis, revealing walking speed, cadence, and stride length as significant indicators of future falls. This finding is in line with our results, although we were not able to analyze the data in a meta-analysis due to the heterogeneity across studies. In recent years, the integration of sensors and wearable devices has gained momentum in fall prediction research. These technologies offer a sensitive approach to measuring gait and balance. As opposed to self-reported responses, wearable devices provide objective and continuous data, allowing for a deeper understanding of a patient’s movement patterns. Recently, wearable sensors have been employed in both healthy individuals and those with PD demonstrating their usefulness to record and analyze gait, balance, and other falls-related risk factors [57, 58]. This advancement promises to enhance the accuracy of fall prediction by capturing subtle changes in gait and balance that may go unnoticed in traditional assessments. However, the multiple parameters derived from sensors introduces complexity in data analysis and more sophisticated analytical techniques might be needed, such as machine learning algorithms, to identify potential predictors for falls.
Regarding the limitations of the current work, one important aspect pertains to the method of fall recording. Falls are self-reported by patients in diaries, which introduces a potential bias and may result in less accurate data on fall incidents. Furthermore, a quantitative analysis of the extracted prognostic factors from the articles was not performed in this study, and, therefore, no statistical data are reported. Combining the current findings with a meta-analysis would provide a higher level of scientific evidence to determine whether factors such as balance or FoG are indeed predictors of falls. However, due to the heterogeneity in the included prognostic factors, confounding factors, and reported outcomes, a descriptive analysis was more suitable. This limitation might be resolved in future studies by focusing on the analysis of specific prognostic factors for falls. Lastly, it is important to note that only prospective studies were included in this review, and there may be additional prognostic factors that have been examined in retrospective longitudinal studies, yielding different results. Nonetheless, the inclusion of prospective studies is deemed valuable, as they offer greater validity in assessing the reliability and predictive value of prognostic factors. However, these studies are usually limited by short follow-up time.
In conclusion, the prognostic factors for falls in PD that were most consistently reported as significant in the literature were previous history of falls, gait disorders, and poor balance. As the prevalence of PD continues to rise globally, elucidating robust prognostic factors for falls is paramount for informing targeted interventions and optimizing patient care. As some prognostic factors have been poorly studied in the literature, such as demographics, and the heterogeneity of confounding factors is high, more research is needed to assess the predictive values of the identified factors. In future studies, using objective instruments like wearable devices for biometric assessment for falls, freezing and gait disorders could enhance the reliability of data collection. Moreover, considering the predictive nature of the intended outcome, future studies could benefit from utilizing more advanced and robust machine learning algorithms to identify predictive factors for falls in PD. This review directs attention towards key variables warranting further investigation for developing tailored fall prevention strategies in PD.
Availability of data
Data used for this systematic review are available upon reasonable request.
References
Allali G, Launay CP, Blumen HM, Callisaya ML, De Cock AM, Kressig RW, Srikanth V, Steinmetz JP, Verghese J, Beauchet O (2017) Falls, cognitive impairment, and gait performance: results from the GOOD initiative. J Am Med Dir Assoc 18:335–340
Kalilani L, Asgharnejad M, Palokangas T, Durgin T (2016) Comparing the incidence of falls/fractures in Parkinson’s disease patients in the US population. PLoS One 11:e0161689
Rodrigues F, Domingos C, Monteiro D, Morouço P (2022) A review on aging, sarcopenia, falls, and resistance training in community-dwelling older adults. Int J Environ Res Public Health 19:874
Lauritzen JB (1996) Hip fractures: incidence, risk factors, energy absorption, and prevention. Bone 18:65s–75s
Walker RW, Chaplin A, Hancock RL, Rutherford R, Gray WK (2013) Hip fractures in people with idiopathic Parkinson’s disease: incidence and outcomes. Movem Disord 28:334–340
Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH (2001) Prospective assessment of falls in Parkinson’s disease. J Neurol 248:950–958
Paul SS, Sherrington C, Canning CG, Fung VS, Close JC, Lord SR (2014) The relative contribution of physical and cognitive fall risk factors in people with Parkinson’s disease: a large prospective cohort study. Neurorehabil Neural Repair 28:282–290
Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L (2017) Falls in Parkinson’s disease: A complex and evolving picture. Movem Disord 32:1524–1536
Kwon K-Y, Park S, Lee EJ, Lee M, Ju H (2021) Association of fall risk factors and non-motor symptoms in patients with early Parkinson’s disease. Sci Rep 11:5171
Latt MD, Lord SR, Morris JG, Fung VS (2009) Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Movem Disord 24:1280–1289
Dennison AC, Noorigian JV, Robinson KM, Fisman DN, Cianci HJ, Moberg P, Bunting-Perry L, Martine R, Duda J, Stern MB (2007) Falling in Parkinson disease: identifying and prioritizing risk factors in recurrent fallers. Am J Phys Med Rehabil 86:621–632
Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N (2005) Falls in outpatients with Parkinson’s disease: frequency, impact and identifying factors. J Neurol 252:1310–1315
Allen NE, Sherrington C, Canning CG, Fung VS (2010) Reduced muscle power is associated with slower walking velocity and falls in people with Parkinson’s disease. Parkinsonism Relat Disord 16:261–264
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Greenhalgh T, Peacock R (2005) Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 331:1064–1065
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5:210
Moons KGM, de Groot JAH, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, Reitsma JB, Collins GS (2014) Critical appraisal and data extraction for systematic reviews of prediction modelling studies: The CHARMS checklist. PLoS Med 11:e1001744
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158:280–286
Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA (2010) Predictors of future falls in Parkinson disease. Neurology 75:116–124
Camicioli R, Majumdar S (2010) Relationship between mild cognitive impairment and falls in older people with and without Parkinson’s disease: 1-Year Prospective Cohort Study. Gait Posture 32:87–91
Geerse DJRM, Marinus J, van Hilten JJ (2019) Walking adaptability for targeted fall-risk assessments. Gait Posture 70:8
Hoskovcová M, Dušek P, Sieger T, Brožová H, Zárubová K, Bezdíček O, Šprdlík O, Jech R, Štochl J, Roth J, Růžička E (2015) Predicting falls in Parkinson disease: what is the value of instrumented testing in OFF medication state? PLoS One 10:e0139849
Gray P, Hildebrand K (2000) Fall risk factors in Parkinson’s disease. J Neurosci Nurs 32:222–228
Ma L, Mi TM, Jia Q, Han C, Chhetri JK, Chan P (2022) Gait variability is sensitive to detect Parkinson’s disease patients at high fall risk. Int J Neurosci 132:888–893
Paul SS, Canning CG, Sherrington C, Lord SR, Close JC, Fung VS (2013) Three simple clinical tests to accurately predict falls in people with Parkinson’s disease. Movem Disord 28:655–662
Sakushima K, Yamazaki S, Fukuma S, Hayashino Y, Yabe I, Fukuhara S, Sasaki H (2016) Influence of urinary urgency and other urinary disturbances on falls in Parkinson’s disease. J Neurol Sci 360:153–157
Schlenstedt C, Brombacher S, Hartwigsen G, Weisser B, Möller B, Deuschl G (2016) Comparison of the fullerton advanced balance scale, mini-BESTest, and berg balance scale to predict falls in Parkinson disease. Phys Ther 96:494–501
van Schooten KS, Taylor ME, Close JCT, Davis JC, Paul SS, Canning CG, Latt MD, Hoang P, Kochan NA, Sachdev PS, Brodaty H, Dean CM, Hulzinga F, Lord SR, Delbaere K (2021) Sensorimotor, cognitive, and affective functions contribute to the prediction of falls in old age and neurologic disorders: an observational study. Arch Phys Med Rehabil 102:874–880
Mak MKAM (2013) The mini-BESTest can predict parkinsonian recurrent fallers: a 6-month prospective study. J Rehabil 45:565–571
Allcock LMRE, Steen IN, Wesnes K, Kenny RA, Burn DJ (2009) Impaired attention predicts falling in Parkinson’s disease. Parkinson’s Relat Disord 15:6
Almeida L, Sherrington C, Allen N, Paul S, Valenca G, Oliveira J, Canning C (2015) Disability is an independent predictor of falls and recurrent falls in people with Parkinson’s disease without a history of falls: a one-year Prospective study. J Parkinsons Dis 5:855–864
Almeida LRVG, Negreiros NN, Pinto EB, Oliveira-Filho J (2016) Comparison of self-report and performance-based balance measures for predicting recurrent falls in people with Parkinson disease: cohort study. Phys Ther 96:11
Beretta VS, Barbieri FA, Orcioli-Silva D, Dos Santos PCR, Simieli L, Vitório R, Gobbi LTB (2018) Can postural control asymmetry predict falls in people with Parkinson’s disease? Mot Control 22:449–461
Cole MHRJ, Naughton GA, Silburn PA (2016) Use of a short-form balance confidence scale to predict future recurrent falls in people with Parkinson disease. Arch Phys Med Rehabil 97:9
Custodio N, Lira D, Herrera-Perez E, Montesinos R, Castro-Suarez S, Cuenca-Alfaro J, Cortijo P (2016) Predictive model for falling in Parkinson disease patients. eNeurologicalSci 5:20–24
Duncan RPCJ, Earhart GM, Ellis TD, Ford MP, Foreman KB, Leddy AL, Paul SS, Canning CG, Thackeray A, Dibble LE (2015) External validation of a simple clinical tool used to predict falls in people with Parkinson disease. Parkinsonism Relat Disord 21:4
LA Duncan RP, Cavanaugh JT, Dibble LE, Ellis TD, Ford MP, Foreman KB, Earhart GM (2013) Comparative utility of the BESTest, mini-BESTest, and brief-BESTest for predicting falls in individuals with Parkinson disease: a cohort study. Phys Ther 93:8
Gazibara T, Kisic-Tepavcevic D, Svetel M, Tomic A, Stankovic I, Kostic VS, Pekmezovic T (2016) Health-related quality of life as a predictor of recurrent falling in Parkinson’s disease: 1-year follow-up study. Psychogeriatrics 16:362–367
Kim JS, Jang W, Cho JW, Ahn JY, Kim HT (2013) Bedside cognitive assessments and falls risk in Parkinson’s disease. Neurol Sci 34:75–78
Mak MK, Pang MY (2009) Fear of falling is independently associated with recurrent falls in patients with Parkinson’s disease: a 1-year prospective study. J Neurol 256:1689–1695
Mak MK, Wong A, Pang MY (2014) Impaired executive function can predict recurrent falls in Parkinson’s disease. Arch Phys Med Rehabil 95:2390–2395
Romagnolo A, Zibetti M, Merola A, Canova D, Sarchioto M, Montanaro E, Artusi C, Vallelonga F, Maule S, Lopiano L (2019) Cardiovascular autonomic neuropathy and falls in Parkinson disease: a prospective cohort study. J Neurol 266:85–91
Venhovens JMJ, Bloem BR, Verhagen WIM (2020) Neurovestibular dysfunction and falls in Parkinson’s disease and atypical parkinsonism: a prospective 1 year follow-up study. Front Neurol. https://doi.org/10.3389/fneur.2020.580285
Wood BH, Bilclough JA, Bowron A, Walker RW (2002) Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry 72:721–725
Gazibara T, Pekmezovic T, Kisic Tepavcevic D, Svetel M, Tomic A, Stankovic I, Kostic VS (2015) Health-related quality of life in patients with Parkinson’s disease: Implications for falling. Parkinsonism Relat Disord 21:573–576
Kataoka H, Tanaka N, Saeki K, Kiriyama T, Ueno S (2014) Low frontal assessment battery score as a risk factor for falling in patients with Hoehn-Yahr stage III Parkinson’s disease: a 2-year prospective study. Eur Neurol 71:187–192
Heinzel S, Maechtel M, Hasmann S, Hobert M, Heger T, Berg D, Maetzler W (2016) Motor dual-tasking deficits predict falls in Parkinson’s disease: a prospective study. Parkinsonism Relat Disord 26:73–77
Lord SGB, Yarnall AJ, Coleman S, Burn D, Rochester L (2016) Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naïve cohort. Movem Disord 31:8
Kataoka H, Ueno S (2015) Low FAB score as a predictor of future falling in patients with Parkinson’s disease: a 2.5-year prospective study. J Neurol 262:2049–2055
Lindholm BBC, Odin P, Hagell P (2021) Longitudinal prediction of falls and near falls frequencies in Parkinson’s disease: a prospective cohort study. J Neurol 268(3):8
Lindholm B, Franzén E, Duzynski W, Odin P, Hagell P (2021) Clinical usefulness of retropulsion tests in persons with mild to moderate Parkinson’s disease. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph182312325
Hiorth YHLJ, Lode K, Pedersen KF (2014) Natural history of falls in a population-based cohort of patients with Parkinson’s disease: an 8-year prospective study. Parkinsonism Relat Disord 20:66
Duncan R, Leddy A, Cavanaugh J, Dibble L, Ellis T, Ford M, Foreman K, Earhart G (2015) Detecting and predicting balance decline in Parkinson disease: a prospective cohort study. J Parkinsons Dis 5:131–139
Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, Gray P, Kerr G, Bloem BR (2007) A meta-analysis of six prospective studies of falling in Parkinson’s disease. Movem Disord 22:1892–1900
Allen NE, Schwarzel AK, Canning CG (2013) Recurrent falls in Parkinson’s disease: a systematic review. Parkinson’s disease 2013:906274
Creaby MW, Cole MH (2018) Gait characteristics and falls in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 57:1–8
Ortega-Bastidas P, Gómez B, Aqueveque P, Luarte-Martínez S, Cano-de-la-Cuerda R (2023) Instrumented timed up and go test (iTUG–more than assessing time to predict falls: a systematic review. Sensors 23:3426
Silva de Lima AL, Evers LJW, Hahn T, Bataille L, Hamilton JL, Little MA, Okuma Y, Bloem BR, Faber MJ (2017) Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: a systematic review. J Neurol 264:1642–1654
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
A.M-G. contributed to the study conception and design. Material preparation, data collection, and analysis were performed by A.M-G. and O.M. The first draft of the manuscript was written by A.M-G. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not received any funding from any institution, including personal relationships, interests, grants, employment, affiliations, patents, inventions, honoraria, consultancies, royalties, stock options/ownership, or expert testimony for the last 12 months.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murueta-Goyena, A., Muiño, O. & Gómez-Esteban, J.C. Prognostic factors for falls in Parkinson’s disease: a systematic review. Acta Neurol Belg 124, 395–406 (2024). https://doi.org/10.1007/s13760-023-02428-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-023-02428-2