Abstract
Despite the large number of studies that have investigated the use of wearable sensors to detect gait disturbances such as Freezing of gait (FOG) and falls, there is little consensus regarding appropriate methodologies for how to optimally apply such devices. Here, an overview of the use of wearable systems to assess FOG and falls in Parkinson’s disease (PD) and validation performance is presented. A systematic search in the PubMed and Web of Science databases was performed using a group of concept key words. The final search was performed in January 2017, and articles were selected based upon a set of eligibility criteria. In total, 27 articles were selected. Of those, 23 related to FOG and 4 to falls. FOG studies were performed in either laboratory or home settings, with sample sizes ranging from 1 PD up to 48 PD presenting Hoehn and Yahr stage from 2 to 4. The shin was the most common sensor location and accelerometer was the most frequently used sensor type. Validity measures ranged from 73–100% for sensitivity and 67–100% for specificity. Falls and fall risk studies were all home-based, including samples sizes of 1 PD up to 107 PD, mostly using one sensor containing accelerometers, worn at various body locations. Despite the promising validation initiatives reported in these studies, they were all performed in relatively small sample sizes, and there was a significant variability in outcomes measured and results reported. Given these limitations, the validation of sensor-derived assessments of PD features would benefit from more focused research efforts, increased collaboration among researchers, aligning data collection protocols, and sharing data sets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by four major motor signs: rest tremor, rigidity, bradykinesia, and postural instability [1]. Non-motor impairments, including executive dysfunctions, memory disturbances, and reduced ability to smell, are also seen in the disease [2–4]. Gait difficulties and balance issues are a disabling problem in many patients with PD, with different contributing factors, such as freezing of gait (FOG), festination, shuffling steps, and a progressive loss of postural reflexes. Its importance is underlined by a high prevalence of fall incidents in PD, especially in the later stages of the disease [5–7].
FOG is defined as a sudden and brief episode of inability to produce effective forward stepping [8]. The phenomenon is closely related to falls, appearing mainly during gait initiation, turning while performing a concomitant concurrent activity (i.e., dual tasks), or approaching narrow spaces [9–13]. Similar to FOG, fall episodes occur mainly during a half-turn or while dual tasking [6]. With disease progression, the increase of FOG and falling episodes, as well as the decrease in effectiveness of dopaminergic therapy amplify the burden related to these symptoms [6, 12, 14].
The management of gait disturbances, such as FOG and falls, often includes pharmacological interventions [12]. However, there is a growing interest in non-pharmacological interventions, such as physiotherapy [15], deep brain stimulation [16], or cueing devices [17, 18]. In all cases, reliable tools are required to determine the severity of gait disorders and evaluate the efficacy of interventions [5].
A number of subjective rating scales are used to evaluate motor symptoms, but most of them have limited validity and reliability [19]. To overcome these limitations, wearable sensors are emerging as new tools to objectively and continuously obtain information about patients’ motor symptoms [20–22]. These sensors, typically consisting of embedded accelerometers, gyroscopes and other, have been used to determine PD-related symptoms, including gait disorders [17, 18, 23–28]. They can act as an extension of health-professionals’ evaluation of PD symptoms, improving treatment, and augmenting self-management [29, 30].
Despite a large number of studies that investigated the use of wearable sensors to detect gait disturbances, such as FOG and falls, there is little agreement regarding the most effective system design, e.g., type of sensors, number of sensors, location of the sensors on the body, and signal processing algorithms. Here, we provide an overview of the use of wearable systems to assess FOG and falls in PD, with emphasis on device setup and results from validation procedures.
Review methodology
A systematic search in the PubMed and Web of Science databases was performed in accordance with the PRISMA statement [31]. These databases were chosen to allow both medical and engineering journals to be included in the search process.
The search query, based on the PICO strategy [31], included Parkinson’s disease representing the Population, wearable, sensors, device representing the Intervention and falls or freezing of gait representing the Comparison. Outcome was not included as a key word to keep the query broad. The truncation symbol (*) and title/abstract filter were used to both broaden the search and provide more specificity. The final search query is shown in Table 1.
The final search was performed in January 2017. In addition to the database search, a search in the references of review articles and book chapters that appeared during the search was performed. The goal was to identify potentially eligible articles absent in the database search.
Articles were selected based upon a set of eligibility criteria. As the objective of this review was to provide an overview of articles published on the topic, selection criteria were kept broad. Therefore, studies were included if they (1) present original research on the validation of wearable sensors (i.e., a single or combination of body worn computer/sensor [32, 33]) to detect, measure or monitor FOG, falls, or fall risk and (2) were performed in Parkinson’s disease patients. Studies were excluded if they (1) only used wearables to deliver cueing for FOG, (2) were published in languages other than English, or (3) did not provide sufficient information about study design and results.
Data extraction was performed using a predefined table. Variables extracted included: author, sample size, device usage (i.e., type of sensor, number of sensors, and location of the device), data collection procedures, and validation results. Validity was considered as the extent to which an instrument is measuring a concept that it is supposed to measure. It can be further divided into different types of validity, such as criterion-referenced validity, construct validity and content validity. In the case of wearable sensors, researchers are often interested in criterion-referenced validity, which can be assessed by the correlation between the sensor-derived outcome and the outcome of a reference instrument that has already been validated [34, 35]. Construct validity, also known as discriminant validity, is commonly used by assessing the extent to which groups that are supposed to produce different outcomes, indeed do so, for example, by comparing PD with non-PD, or DBS ON with DBS OFF.
Results
Selection process
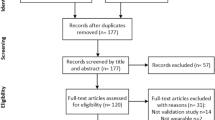
In total, 552 articles were retrieved by the query. The selection process led to the final inclusion of 27 articles. Of those, 23 articles related to FOG, and 4 to falls. A complete overview of the selection process is presented in Fig. 1.
Methodologies
FOG detection
A total of 23 articles investigated the use of wearable sensors to assess FOG in PD [18, 28, 36–56] (Table 2). The sample sizes varied from 1 [28] to 48 PD [51] per study, with a non-PD group being included in a few studies [28, 40, 48, 51, 53, 56]. Disease severity, when reported, ranged from 2 to 4 according to the Hoehn and Yahr scale. Data were collected according to three types of protocols: (1) a set of structured tasks performed in a laboratory environment (n = 18); (2) a protocol performed in a laboratory environment in which at least a part of which was designed to capture naturalistic behaviour (n = 2); and (3) natural or naturalistic behaviour in a home environment (n = 3).
The types of sensors embedded in the devices worn by the participants varied. Tri-axial accelerometers were used in 22 articles, either as a single sensor (48%, n = 11), or combined with gyroscopes (35%, n = 8), or magnetometers (13%, n = 3). One study used electroencephalogram to measure changes in the brain activity from pre-determined areas during FOG episodes. Regarding the number of body locations, 56% (n = 13) of the studies utilized one location, while the other 44% (n = 10) used a combination of two or more locations. The shin (66% of studies, n = 16; 4 times used as the single location) and waist (33% of studies, n = 8; 3 times as the single location) were the most common body locations for the devices, although nine other locations were also explored (Fig. 2).
Falls: detection and fall risk analysis
Four articles on falls were retrieved: one article on fall detection and three articles presented the use of wearable sensors for analyzing fall risk. All protocols were performed in a home-based setting (Table 3) [57–60], and the sample size varied from one patient in a case report [57] up to 107 PD in a cross-sectional study [59]. One study reported disease severity and had an average Hoehn and Yahr score of 2.6 ± 0.7 [59]. All studies used tri-axial accelerometers. One study combined this sensor with force and bending sensors [58]; another with gyroscopes [60]. Sensor body locations included chest, insole (i.e., under the arch of the foot), and lower back.
Validation
FOG detection
Among the 23 articles investigating FOG detection, 18 reported measures of validation performance (e.g., sensitivity, specificity, or accuracy) [17, 36–45, 47–49, 52–55], three studies used correlation measures, correlating the wearable-derived measure with the period of freezing or number of FOG events [50, 51, 56], and two studies did not report validity measures [28, 46].
Overall, validity values ranged from 73 to 100% for sensitivity, and from 67 to 100% for specificity, and accuracy ranged from 68% up to 96%. Validity measures are summarized and compared across protocol setups in Figs. 3 and 4.
Fall detection and fall risk analysis
One article investigated the use of wearable sensors to detect falls, by comparing the data from a self-reported diary to the sensor data. The sensor captured 19 fall events from a total of 22 self-reported events [57].
Three articles presented the use of wearable sensors for analyzing fall risk. All of them reported discriminant validity by comparing sensor-derived outcomes between different groups, such as fallers and non-fallers or PD versus non-PD (see Table 3 for details). Weiss et al. [59] reported an illustrative approach, whereby the 107 participating PD patients wore one sensor in the lower back and made diary annotations about fall events. The sensor data, collected remotely in the patient’s home, were subsequently used to calculate a fall risk index. The time until first fall was significantly lower in subjects with a higher variable gait pattern (log rank test: p = 0.0018, Wilcoxon test: p = 0.0014).
Discussion
This review included 27 articles, 23 on FOG, and four on falls. FOG studies were performed either in a laboratory or at home, with different types of protocols (structured versus free-movement). The shin (16/28 studies) was the most common device location and tri-axial accelerometers (26/28 studies) the most common sensor type. Sensitivity ranged from 73% to 100% and specificity ranged from 67% to 100% for the detection of FOG. Fall and fall risk studies were all home-based, using mostly one device (3/4 studies) containing tri-axial accelerometers. Sensors were positioned on the chest, insole, and lower back. The systems detected falls or quantified fall risk by various approaches and with varying degrees of validity.
FOG detection
The results in this review support the potential for wearable devices. In the laboratory, systems showed a moderate to high specificity and sensitivity, which are in line with other evidence that wearable systems detecting FOG are already well validated in a laboratory setting [30]. Moreover, promising results were also achieved in studies performed in the home environment. Interestingly, the comparison of validity measures in terms of sensitivity and specificity (Figs. 3, 4) suggests that wearable sensors are able to accurately detect FOG, independent of study protocol (e.g., home versus laboratory environment; structured versus unstructured protocols) and system design (e.g., one sensor only versus multiple sensors, and one device versus a set of combined devices in different body locations). However, one should be cautious when directly comparing reported performance between studies, for a number of reasons: in particular, one should consider additional factors, such as algorithm used, outcome definitions, data analysis methods, and the intended application of the system.
First, even though FOG is a well-defined symptom [8], what objectively constitutes FOG is unclear. The challenge lies in rigorously defining, from an algorithmic point of view, such a complex event, which can appear in different forms and intensities. Furthermore, the definition of the measured outcome has an important impact upon instrument validity assessment. In this review, some studies only included long-duration FOG episodes. Omitting small FOG episodes may lead to inaccurate estimates of FOG detection rates. A comprehensive definition such as that used by Djuric–Jovici and colleagues [47], differentiating between FOG with trembling and FOG with complete motor blocks prior to video labeling and test properties, seems to address the problem by incorporating different types of FOG events. However, this definition was not used in other studies. A clear and comprehensive definition would improve the comparability of instrument performance.
Second, the intended application of the instrument is another aspect to be considered in FOG detection. It is attractive to aim for rates of 100% specificity and sensitivity. However, this may result in signal processing operations which require substantial computational resources. As illustrated by Ahlrichs [37], the detection of FOG episodes was achieved with high sensitivity and specificity, but the data processing was time-consuming with delays of up to 60 s. Similarly, algorithms with high accuracy may require substantial computational resources which may have an adverse effect on power consumption and hence battery life for non-intrusive, portable devices. This fact may prevent the use of such systems for real-time detection and cueing. Therefore, it is reasonable to conclude that at this point, the acceptability of instrument performance in detection of FOG relate to its application, and many of these algorithms will require substantial mathematical and engineering efforts in order to reduce computational delays to an acceptable level. Furthermore, some algorithms required individual calibration and others did not, which also has practical consequences for applications in clinical and research practice.
Finally, although there exists the potential for these instruments being applied to long-term monitoring in free living conditions, only a few systems were actually validated in the home environment. Therefore, the majority of the technology available lacks “ecological” validation. Thus, further research using larger sample sizes, longer follow-up periods under more realistic home environments is necessary.
Fall detection and fall risk calculation
Del Din and colleagues described that real-world detection of falls is a substantial challenge from a technical perspective, and almost all evidence in their review was limited to controlled settings and young healthy adults [30]. This finding is confirmed in this review, most clearly illustrated by the fact that we only found one article reporting on fall detection accuracy in PD. However, it is possible that this small number of articles is not only a result of the complexity of capturing falls in PD under realistic, free-living conditions. It certainly highlights an area where the validity of wearable sensors still needs to be examined. In addition, fall risk calculation has the potential to provide objective information before the fall event happens, which may be more valuable than simply counting the number of events and dealing with the consequences.
Fall risk estimation has a clear relevance for clinical practice [58]. Falls are common and disabling, even in early PD [61]. In addition, falls are also related to physical injury [61], high hospitalization cost [62], and social/psychological impact [63], either on their own or due to the anticipatory fear of falling [64]. Even though the number of retrieved articles investigating fall risk calculation was not high, the results seem to confirm the potential for wearable sensors to accurately calculate fall risk for PD.
Conclusion
This systematic review presents an overview of studies investigating the use of wearable sensors for FOG and falls in Parkinson’s disease. Despite promising validation initiatives, study sample sizes are relatively small, participants are mainly in early stages of the disease, protocols are largely laboratory-based, and there is little consensus on algorithms analysis. Further work in ecological validation, in free-living situations, is necessary. There also is a lack of consistency in outcomes measured, methods of assessing validity, and reported results. Given these limitations, the validation of sensor-derived assessments of PD features would benefit from increased collaboration among researchers, aligning data collection protocols, and sharing data sets.
References
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. doi:10.1136/jnnp.2007.131045
Gratwicke J, Jahanshahi M, Foltynie T (2015) Parkinson’s disease dementia: a neural networks perspective. Brain:awv104
Levin BE, Katzen HL (1995) Early cognitive changes and nondementing behavioral abnormalities in Parkinson’s disease. Adv Neurol 65:85–95
Dickson DW (2012) Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med 2(8):a009258
Chen P-H, Wang R-L, Liou D-J, Shaw J-S (2013) Gait disorders in Parkinson’s disease: assessment and management. Int J Gerontol 7(4):189–193
Grabli D, Karachi C, Welter M-L, Lau B, Hirsch EC, Vidailhet M, François C (2012) Normal and pathological gait: what we learn from Parkinson’s disease. J Neurol Neurosurg Psychiatry 83(10):979–985
Roller WC, Glatt S, Vetere-Overfield B, Hassanein R (1989) Falls and Parkinson’s disease. Clin Neuropharmacol 12(2):98–105
Giladi N, Nieuwboer A (2008) Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord 23(S2):S423–S425
Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A (2011) Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 10(8):734–744. doi:10.1016/S1474-4422(11)70143-0
Browner N, Giladi N (2010) What can we learn from freezing of gait in Parkinson’s Disease? Curr Neurol Neurosci Rep 10(5):345–351. doi:10.1007/s11910-010-0127-1
Virmani T, Moskowitz CB, Vonsattel J-P, Fahn S (2015) Clinicopathological characteristics of freezing of gait in autopsy-confirmed Parkinson’s disease. Mov Disord 30(14):1874–1884. doi:10.1002/mds.26346
Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N, Bloem BR (2015) Freezing of gait: a practical approach to management. Lancet Neurol 14(7):768–778
Okuma Y (2014) Freezing of gait and falls in Parkinson’s disease. J Parkinson’s Dis 4(2):255–260
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney multicenter study of Parkinson’s disease: Non-L-dopa–responsive problems dominate at 15 years. Mov Disord 20(2):190–199
Nieuwboer A (2008) Cueing for freezing of gait in patients with Parkinson’s disease: a rehabilitation perspective. Mov Disord 23(S2):S475–S481
Ferraye MU, Debû B, Pollak P (2008) Deep brain stimulation effect on freezing of gait. Mov Disord 23(S2):S489–S494
Bachlin M, Plotnik M, Roggen D, Giladi N, Hausdorff JM, Troster G (2010) A wearable system to assist walking of Parkinson s disease patients. Methods Inf Med 49(1):88–95. doi:10.3414/me09-02-0003
Bachlin M, Plotnik M, Roggen D, Maidan I, Hausdorff JM, Giladi N, Troster G (2010) Wearable assistant for Parkinson’s disease patients with the freezing of gait symptom. IEEE Trans Inf Technol Biomed 14(2):436–446. doi:10.1109/titb.2009.2036165
Ebersbach G, Baas H, Csoti I, Müngersdorf M, Deuschl G (2006) Scales in Parkinson’s disease. J Neurol 253(4):iv32–iv35. doi:10.1007/s00415-006-4008-0
Ossig C, Antonini A, Buhmann C, Classen J, Csoti I, Falkenburger B, Schwarz M, Winkler J, Storch A (2016) Wearable sensor-based objective assessment of motor symptoms in Parkinson’s disease. J Neural Transm 123(1):57–64. doi:10.1007/s00702-015-1439-8
Chen B-R, Patel S, Buckley T, Rednic R, McClure DJ, Shih L, Tarsy D, Welsh M, Bonato P (2011) A web-based system for home monitoring of patients with Parkinson’s disease using wearable sensors. IEEE Trans Biomed Eng 58(3):831–836
Patel S, Chen BR, Buckley T, Rednic R, McClure D, Tarsy D, Shih L, Dy J, Welsh M, Bonato P (2010) Home monitoring of patients with Parkinson’s disease via wearable technology and a web-based application. In: Annual International Conference of the IEEE Engineering in Medicine and Biology. IEEE, pp 4411–4414
Sharma V, Mankodiya K, De La Torre F, Zhang A, Ryan N, Ton TG, Gandhi R, Jain S (2014) SPARK: Personalized Parkinson Disease Interventions through Synergy between a Smartphone and a Smartwatch. In: Design, user experience, and usability. User experience design for everyday life applications and services. Springer, New York, pp 103–114
Patel S, Lorincz K, Hughes R, Huggins N, Growdon J, Standaert D, Akay M, Dy J, Welsh M, Bonato P (2009) Monitoring motor fluctuations in patients with Parkinson’s disease using wearable sensors. Inf Technol Biomed IEEE Trans 13(6):864–873
Griffiths RI, Kotschet K, Arfon S, Xu ZM, Johnson W, Drago J, Evans A, Kempster P, Raghav S, Horne MK (2012) Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J Parkinson’s Dis 2(1):47–55
Cole BT, Roy SH, Nawab SH (2011) Detecting freezing-of-gait during unscripted and unconstrained activity. In: Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE. pp 5649–5652. doi:10.1109/IEMBS.2011.6091367 (30 Aug 2011–3 Sept 2011)
Djurić-Jovičić M, Jovičić NS, Milovanović I, Radovanović S, Kresojević N, Popović MB (2010) Classification of walking patterns in Parkinson’s disease patients based on inertial sensor data. In: Neural Network Applications in Electrical Engineering (NEUREL), 2010 10th Symposium on, 23–25 Sept. 2010, pp 3–6. doi:10.1109/NEUREL.2010.5644040
Jovanov E, Wang E, Verhagen L, Fredrickson M, Fratangelo R (2009) deFOG--a real time system for detection and unfreezing of gait of Parkinson’s patients. In: Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference, pp 5151–5154. doi:10.1109/iembs.2009.5334257
Patel S, Park H, Bonato P, Chan L, Rodgers M (2012) A review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil 9(1):1
Del Din S, Godfrey A, Mazzà C, Lord S, Rochester L (2016) Free-living monitoring of Parkinson’s disease: lessons from the field. Mov Disord 31(9):1293–1313
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151(4):W65–W94
Billinghurst M, Starner T (1999) Wearable devices: new ways to manage information. Computer 32(1):57–64
Kubota KJ, Chen JA, Little MA (2016) Machine learning for large-scale wearable sensor data in Parkinson’s disease: concepts, promises, pitfalls, and futures. Mov Disord 31(9):1314–1326
Bassett DR Jr, Rowlands AV, Trost SG (2012) Calibration and validation of wearable monitors. Med Sci Sports Exerc 44(1 Suppl 1):S32
Higgins PA, Straub AJ (2006) Understanding the error of our ways: mapping the concepts of validity and reliability. Nurs Outlook 54(1):23–29. doi:10.1016/j.outlook.2004.12.004
Martín DR, Samà A, Pérez-López C, Català A, Mestre B, Alcaine S, Bayés À (2016) Comparison of features, window sizes and classifiers in detecting freezing of gait in patients with Parkinson’s disease through a Waist-Worn Accelerometer
Ahlrichs C, Sama A, Lawo M, Cabestany J, Rodriguez-Martin D, Perez-Lopez C, Sweeney D, Quinlan LR, Laighin GO, Counihan T, Browne P, Hadas L, Vainstein G, Costa A, Annicchiarico R, Alcaine S, Mestre B, Quispe P, Bayes A, Rodriguez-Molinero A (2016) Detecting freezing of gait with a tri-axial accelerometer in Parkinson’s disease patients. Med Biol Eng Comput 54(1):223–233. doi:10.1007/s11517-015-1395-3
Tzallas AT, Tsipouras MG, Rigas G, Tsalikakis DG, Karvounis EC, Chondrogiorgi M, Psomadellis F, Cancela J, Pastorino M, Arredondo Waldmeyer MT, Konitsiotis S, Fotiadis DI (2014) PERFORM: a system for monitoring, assessment and management of patients with Parkinson’s disease. Sensors 14(11):21329–21357. doi:10.3390/s141121329
Mazilu S, Blanke U, Hardegger M, Tröster G, Gazit E, Hausdorff JM (2014) GaitAssist: a daily-life support and training system for parkinson’s disease patients with freezing of gait. In: Proceedings of the 32nd annual ACM conference on Human factors in computing systems. ACM, New York, pp 2531–2540
Cole BT, Roy SH, Nawab SH (2011) Detecting freezing-of-gait during unscripted and unconstrained activity. In: Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference, pp 5649–5652. doi:10.1109/iembs.2011.6091367
Rezvanian S, Lockhart TE (2016) Towards real-time detection of freezing of gait using wavelet transform on wireless accelerometer data. Sensors. doi:10.3390/s16040475
Zach H, Janssen AM, Snijders AH, Delval A, Ferraye MU, Auff E, Weerdesteyn V, Bloem BR, Nonnekes J (2015) Identifying freezing of gait in Parkinson’s disease during freezing provoking tasks using waist-mounted accelerometry. Parkinsonism Relat Disord 21(11):1362–1366. doi:10.1016/j.parkreldis.2015.09.051
Kim H, Lee HJ, Lee W, Kwon S, Kim SK, Jeon HS, Park H, Shin CW, Yi WJ, Jeon BS, Park KS (2015) Unconstrained detection of freezing of gait in Parkinson’s disease patients using smartphone. In: Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference, pp 3751–3754. doi:10.1109/embc.2015.7319209
Coste CA, Sijobert B, Pissard-Gibollet R, Pasquier M, Espiau B, Geny C (2014) Detection of freezing of gait in Parkinson disease: preliminary results. Sensors (Basel, Switzerland) 14(4):6819–6827. doi:10.3390/s140406819
Kwon Y, Park SH, Kim JW, Ho Y, Jeon HM, Bang MJ, Jung GI, Lee SM, Eom GM, Koh SB, Lee JW, Jeon HS (2014) A practical method for the detection of freezing of gait in patients with Parkinson’s disease. Clin Interv Aging 9:1709–1719. doi:10.2147/cia.s69773
Yungher DA, Morris TR, Dilda V, Shine J, Naismith SL, Lewis SJG, Moore ST (2014) Temporal characteristics of high-frequency lower-limb oscillation during Freezing of Gait in Parkinson’s Disease. Parkinsons Dis. doi:10.1155/2014/606427
Djuric-Jovicic MD, Jovicic NS, Radovanovic SM, Stankovic ID, Popovic MB, Kostic VS (2014) Automatic identification and classification of freezing of gait episodes in Parkinson’s disease patients. IEEE Trans Neural Syst Rehabil Eng 22(3):685–694. doi:10.1109/tnsre.2013.2287241
Tripoliti EE, Tzallas AT, Tsipouras MG, Rigas G, Bougia P, Leontiou M, Konitsiotis S, Chondrogiorgi M, Tsouli S, Fotiadis DI (2013) Automatic detection of freezing of gait events in patients with Parkinson’s disease. Comput Methods Programs Biomed 110(1):12–26. doi:10.1016/j.cmpb.2012.10.016
Moore ST, Yungher DA, Morris TR, Dilda V, MacDougall HG, Shine JM, Naismith SL, Lewis SJ (2013) Autonomous identification of freezing of gait in Parkinson’s disease from lower-body segmental accelerometry. J Neuroeng Rehabil 10:19. doi:10.1186/1743-0003-10-19
Morris TR, Cho C, Dilda V, Shine JM, Naismith SL, Lewis SJ, Moore ST (2012) A comparison of clinical and objective measures of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 18(5):572–577
Mancini M, Priest KC, Nutt JG, Horak FB (2012) Quantifying freezing of gait in Parkinson’s disease during the instrumented timed up and go test. In: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE, pp 1198–1201
Niazmand K, Tonn K, Zhao Y, Fietzek U, Schroeteler F, Ziegler K, Ceballos-Baumann A, Lueth T (2011) Freezing of Gait detection in Parkinson’s disease using accelerometer based smart clothes. In: 2011 IEEE Biomedical Circuits and Systems Conference (BioCAS). IEEE, pp 201–204
Moore ST, MacDougall HG, Ondo WG (2008) Ambulatory monitoring of freezing of gait in Parkinson’s disease. J Neurosci Methods 167(2):340–348. doi:10.1016/j.jneumeth.2007.08.023
Handojoseno AM, Gilat M, Ly QT, Chamtie H, Shine JM, Nguyen TN, Tran Y, Lewis SJ, Nguyen HT (2015) An EEG study of turning freeze in Parkinson’s disease patients: the alteration of brain dynamic on the motor and visual cortex. In: Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference, pp 6618–6621. doi:10.1109/embc.2015.7319910
Capecci M, Pepa L, Verdini F, Ceravolo MG (2016) A smartphone-based architecture to detect and quantify freezing of gait in Parkinson’s disease. Gait Posture 50:28–33. doi:10.1016/j.gaitpost.2016.08.018
Mancini M, Smulders K, Cohen RG, Horak FB, Giladi N, Nutt JG (2016) The clinical significance of freezing while turning in Parkinson’s disease. Neuroscience 343:222–228. doi:10.1016/j.neuroscience.2016.11.045
Tamura T (2005) Wearable accelerometer in clinical use. In: Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference, vol 7, pp 7165–7166. doi:10.1109/iembs.2005.1616160
Ayena J, Zaibi H, Otis M, Menelas BA (2015) Home-Based Risk of Falling Assessment Test Using a Closed-Loop Balance Model. IEEE Trans Neural Syst Rehabil Eng. doi:10.1109/tnsre.2015.2508960
Weiss A, Herman T, Giladi N, Hausdorff JM (2014) Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PLoS ONE 9(5):e96675. doi:10.1371/journal.pone.0096675
Iluz T, Gazit E, Herman T, Sprecher E, Brozgol M, Giladi N, Mirelman A, Hausdorff JM (2014) Automated detection of missteps during community ambulation in patients with Parkinson’s disease: a new approach for quantifying fall risk in the community setting. J Neuroeng Rehabil 11:48. doi:10.1186/1743-0003-11-48
Bloem BR, Grimbergen YAM, Cramer M, Willemsen M, Zwinderman AH (2001) Prospective assessment of falls in Parkinson’s disease. J Neurol 248(11):950–958. doi:10.1007/s004150170047
Alexander BH, Rivara FP, Wolf ME (1992) The cost and frequency of hospitalization for fall-related injuries in older adults. Am J Public Health 82(7):1020–1023. doi:10.2105/AJPH.82.7.1020
Bloem BR, Hausdorff JM, Visser JE, Giladi N (2004) Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord 19(8):871–884
Adkin AL, Frank JS, Jog MS (2003) Fear of falling and postural control in Parkinson’s disease. MovDisord 18(5):496–502. doi:10.1002/mds.10396
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Ana Lígia Silva de Lima is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES (Grant Number 0428-140). Luc J. W. Evers is supported by a Research Grant provided by UCB and Philips Research. Tim Hahn is supported by a Research Grant provided by Stichting Parkinson Fonds. Lauren Bataille and Jamie L. Hamilton are supported by the Michael J. Fox Foundation. Max A. Little received Research funding support from the Michael J. Fox Foundation and UCB. Yasuyuki Okuma has no conflict of interest. Bastiaan R. Bloem received Grant support from the Michael J. Fox Foundation and Stichting Parkinson Fonds. Marjan J. Faber received Grant support from the Michael J. Fox Foundation, Stichting Parkinson Fonds and Philips Research.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Silva de Lima, A.L., Evers, L.J.W., Hahn, T. et al. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: a systematic review. J Neurol 264, 1642–1654 (2017). https://doi.org/10.1007/s00415-017-8424-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8424-0