Abstract
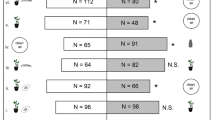
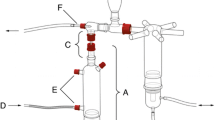
The search behavior and parasitism of trichogrammatids can be affected by volatile compounds emitted by plants under herbivory and/or oviposition. Our aim was to evaluate the chemotactic behavior and parasitism rates of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) females against two varieties of corn and one of rice that underwent herbivory or oviposition by Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). In a glass Y-tube olfactometer, the parasitoids were given the choice between plants damaged by either herbivory or oviposition, with and without sentinel eggs, against those without damage. We also evaluated the average of parasitized eggs and the parasitoid emergence in sentinel eggs, which were next to plants that underwent herbivory contrasted with eggs next to undamaged plants. Trichogramma pretiosum was more attracted to rice and corn plants evaluated 24 h after herbivory compared to undamaged plants. Parasitoids preferred oviposited rice plants over control plants. Oviposited corn plants after 48 h were more attractive than non-oviposited plants without sentinel eggs. In the presence of sentinel eggs on the olfactometer tests, there was no difference in oviposition preference in corn. Parasitism was higher in sentinel eggs located near plants damaged by herbivory. This suggested that the egg parasitoid T. pretiosum not only uses chemical clues from rice and corn plants, damaged by herbivory, but also uses them as a strategy to search and increase parasitism in S. frugiperda eggs. However, the results of oviposition tests showed that plants of different species and varieties might respond differently to this type of damage.





Similar content being viewed by others
References
Acevedo FE, Smith P, Peiffer M, Helms A, Tokker J, Felton GW (2019) Phytohormones in fall armyworm saliva modulate defense responses in plants. J Chem Ecol. https://doi.org/10.1007/s10886-019-01079-z
Arimura GI, Kost C, Boland W (2005) Herbivore-induced, indirect plant defenses. Bioch. Bioph Acta 1734:91–111
Beserra EB, Dias CTS, Parra JRP (2002) Distribution and parasitism of Spodoptera frugiperda (Lepidoptera: Noctuidae) eggs different phenological stages of corn. Florida Entomol 85:588–593
Bruce TJ, Midega CA, Birkett MA, Pickett JA, Khan ZR (2010) Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol Lett 6:314–7
Colazza S, Fucarino A, Peri E, Salermo G, Conti E, Bin F (2004) Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J Exp Biol 207:47–53
Counce PA, Keisling TC, Mitchell AJ (2000) A uniform, objective, and adaptive system for expressing rice development. Crop Sci 40(2):436–443
Cruz I (1995) A lagarta-do-cartucho na cultura do milho. Embrapa-CNPMS, Sete Lagoas 45p.
Degen T, Dillmann C, Poll FM, Turlings TCJ (2004) High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol 135(4):1928–1938
Dequech STB, Camera C, Sturza VS, Ribero LP, Querino BR, Poncio S (2013) Population fluctuation of Spodoptera frugiperda eggs and natural parasitism by Trichogramma in maize. Acta Sci 35:295–300
Dudareva N, Negre F, Nagegowda DA, Orlova I (2006) Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci 25:417–240
Fatouros N, Bukovinskine’Kiss G, Kalkrs LA, Gamborena RS, Dicke M, Hilker M (2005) Oviposition-induced plant cues: do they arrest Trichogramma wasps during host location? Entom Exp Appl 115(1):207–215
Fatouros N, Pachalidou FG, Cordero WVA, Van Loon A, Nunn R, Dicke M, Hilker M, Huigens ME (2009) Anti-aphrodisiac compounds of male butterflies increase the risk of egg parasitoid attack by inducing plant synomone production. J Chem Ecol 35:1373–1381
Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, Van Loon JJA, Dicke M, Harvey JA, Gols R, Huingens M (2012) E. Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS One 7:43607
Figueredo MLC, Cruz I, Silva RB, Foster JE (2015) Biological control with Trichogramma pretiosum increases organic maize productivity by 19.4%. Agron Sustain Dev 35(3):1175–1183
Geiselhardt S, Yoneya K, Blenn B, Drechslern GJ, Kunze R, Hilker M (2013) Egg laying of Cabbage White Butterfly (Pieris brassicae) on Arabidopsis thaliana affects subsequent performance of the larvae. PLoS One 8(3):e59661
Guo H, Wang CZ (2019) The ethological significance and olfactory detection of herbivore induced plant volatiles in interactions of plants, herbivorous insects, and parasitoids. Arthropod Plant Interact 13:161–179
Gurr GM, Kvedaras OL (2010) Synergizing biological control: scope for sterile insect technique, induced plant defences and cultural techniques to enhance natural enemy impact. Biol Control 52(3):198–207
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178(1):41–61
Hilker M, Meiners T (2010) How do plants “notice” attack by herbivorous arthropods? Biol Rev Camb Philos Soc 85:267–280
Hoballah MEFH, Tamo C, Turlings TCJ (2002) Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: is quality or quantity important? J Chem Ecol 28:951–968
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Jurat-Fuentes JL, Crickmore N (2017) Specificity determinants for Cry insecticidal proteins: insights from their mode of action. J Invertebr Pathol 142:5–10
Ko K, Liu Y, Hou M, Babendreier D, Zhang F, Song K (2014) Evaluation for potential Trichogramma (Hymenoptera: Trichogrammatidae) strains for control of the striped stem borer (Lepidoptera: Crambidae) in the greater mekong subregion. J Econ Entomol 107:955–963
Lopes FB, Sant’Ana J (2018) Responses of Spodoptera frugiperda and Trichogramma pretiosum to rice plants exposed to herbivory and phytohormones. Neotrop Entomol 48:381–390
Moreira LSD (2010) Expressão gênica e voláteis induzidos pela herbivoria de Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) em milho, Zea mays L. (Poaceae). 2010. Dissertação (Mestrado em Entomologia) – Escola Superior de Agricultura “Luiz de Queiros”, Piracicaba.
Mattiacci L, Dicke M, Posthumus MA (1995) Beta-glucosidase - an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci U S A 92:2036–2040
Michereff MFF, Laumann RA, Borges M, Michereff Filho M, Diniz IR, Farias Neto AL, Moraes MCB (2011) Volatiles mediating plant-herbivory-natural enemy interaction in resistant and susceptible soybean cultivars. J Chem Ecol 37:273–285
Michereff MF, Magalhães DM, Hassemer MJ, Laumann RA, Zhou J, Ribeiro PEA, Viana PA, Guimarães PEO, Schimmel PHC, Borges M, Picketts JA, Birkett MA, Moraes MCB (2019) Variability in herbivore-induced defence signalling across different maize genotypes impacts significantly on natural enemy foraging behaviour. J Pest Sci 92:723–736
Noldus LPJJ (1989) Semiochemicals, foraging behaviour and quality of entomophagous insects for biological control. J Appl Entomol 108:425–451
Parra JRP (1997) Técnicas de criação de Anagasta kuehniella, hospedeiro alternativo para a produção de Trichogramma. In: Parra JRP, Zucchi RA (eds) Trichogramma e o controle biológico aplicado. Piracicaba, FEALQ, pp 121–150
Parra, J. R. P. (2001). Técnicas de criação de insetos para programas de controle biológico: Piracicaba: FEALQ.
Peñaflor MFGV, Erb M, Miranda LA, Werneburg AL, Bento JMS (2011) Herbivore-induced plant volatiles serve as host location cues for a generalist and a specialist egg parasitoid. J Chem Ecol 37:1304–1313
Querino RB, Silva NNP, Zucchi RA (2016) Natural parasitism by Trichogramma spp. in agroecosystems of the Mid-North, Brazil. Cienc Rural 46:1521–152377
Ritchie SW, Hanway JJ, Benson GO (1992) How a corn plant develops. Special Report 48, Iowa State University, Ames, IA, USA
Sá LA, Parra JRP (1994) Natural parasitism of Spodoptera frugiperda and Helicoverpa zea (Lepidoptera: Noctuidae) eggs in corn by Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Florida Entomol 77:185–188
Tamiru A, Bruce TJA, Woodcock CM, Caulfield JC, Midegal CAO, Ogol CKPO, Mayon P, Birkett MA, Pickett JA, Khan ZR (2011) Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol Lett 14:1075–1083
Tognon R, Aldrich JR, Buffington ML, Talamas EJ, Sant’Ana J, Zalom FG (2017) Halyomorpha halys (Heteroptera: Pentatomidae) egg surface chemicals inhibit North American Telenomus and Trissolcus (Hymenoptera: Scelionidae) parasitism. Biol Control 114:39–44
Tooker JF, Demoraes CM (2005) Jasmonate in lepidopteran eggs and neonates. J Chem Ecol 31:2753–2759
Tooker JF, Demoraes CM (2006) Jasmonate, Salicylate, and benzoate in insect eggs. J Chem Ecol 33:331–343
Tumlinson JH, Turlings TCJ, Lewis WJ (1993) Semiochemically mediated foraging behavior in beneficial parasitic insects. Arch Insect Biochem Physiol 22:385–391
Turlings TC, Alborn HT, Loughrin JH, Tumlison JH (2000) Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera exigua: isolation and bioactivity. J Chem Ecol 26:1573–1561
Vet LEM, Dicke M (1992) Ecology of infochemicals used by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Vinson SB (1998) The general host seletion behavior of parasitoid hymenoptera a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol Control 11:79–96
Xavier LMS, Laumann RA, Borges M, Magalhães DM, Vilela EF, Moraes MCB (2011) Trichogramma pretiosum attraction due to the Elasmopalpus lignosellus damage in maize. Pesq Agropec Bras 46:578–585
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
War AR, Paulraj MG, Buhrroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7(10):1306–1320
Yuan JS, Kollner TG, Wiggins G, Grant J, Degenhardt J, Chen F (2008) Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J 55:491–503
Funding
We would like to thank the Coordinating Committee on the Improvement of Higher Education Personnel (CAPES) of Brazil for providing the fellowship to the first author and the National Council for Scientific and Technological Development for the financial support for fellowships awarded to second author (CNPq 303758/2018-0).
Author information
Authors and Affiliations
Contributions
TCSS and JS conceived and designed the study; TCSS and NAL performed the experiments and analyzed the data; TCSS, NAL, and JS wrote the manuscript.
Corresponding author
Additional information
Edited by Gabriel Manrique
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sousa, T.C.d., Leite, N.A. & Sant’Ana, J. Responses of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) to Rice and Corn Plants, Fed and Oviposited by Spodoptera frugiperda (Lepidoptera: Noctuidae). Neotrop Entomol 50, 697–705 (2021). https://doi.org/10.1007/s13744-021-00876-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-021-00876-0